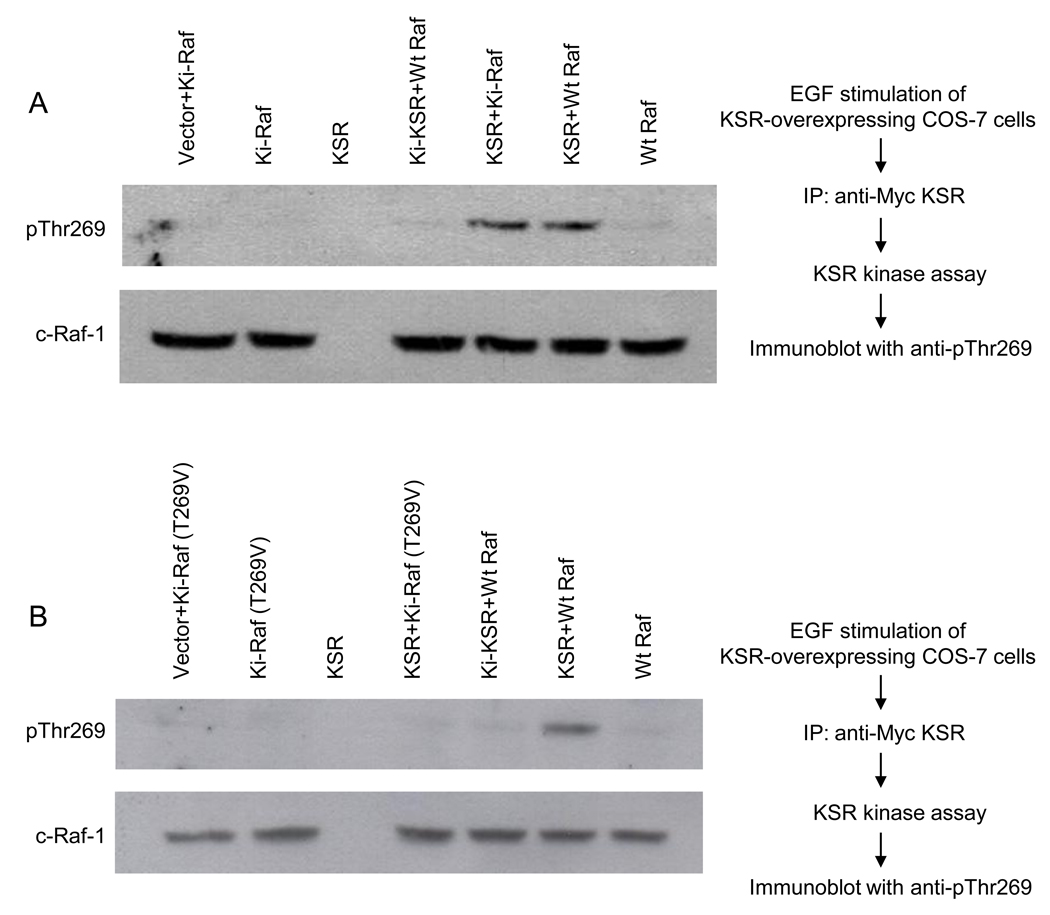
Fig. 3. EGF-stimulated KSR1 enhances phosphorylation of purified recombinant c-Raf-1 at Thr269 in vitro.
COS-7 cells, transfected with mouse Myc-KSR1, were treated with 10ng/ml EGF for 3min at 37°C. Myc-KSR1 was immunoprecipitated from 1mg cell lysate, purified to near homogeneity, and employed to phosphorylate human FLAG-c-Raf-1 as described in Supplementary Methods. (A) KSR1 phosphorylates kinase inactive Ki-c-Raf-1(K375M). Immunoprecipitated KSR1 was incubated in a reaction mixture containing 100µM ATP and either purified FLAG-c-Raf-1 or FLAG-Ki-c-Raf-1(K375M), while Ki-KSR1 reactions contained Wt c-Raf-1. After 60min incubation, phosphorylated c-Raf-1 proteins were resolved by 8% SDS-PAGE and detected by western blotting using anti-pThr269 antibody. Lower panel shows c-Raf-1 loading levels. (B) KSR1 does not phosphorylate Ki-c-Raf-1 substituted at Thr269 with valine. These studies, using Ki-c-Raf-1(T269V/K375M) as substrate, were performed as in (A). Data depict 1 of 3 experiments for (A–B).