Abstract
Cochlear spiral ganglion neurons (SGN) provide the only pathway for transmitting sound evoked activity from the hair cells to the central auditory system. Neurotrophic factor-3 (NT-3) and brain derived neurotrophic factor (BDNF) released from hair cells and supporting cells exert a profound effect on SGN survival and neural firing patterns; however, it is unclear what the effects NT-3 and BDNF have on the type of neurotransmitter receptors expressed on SGN. To address this question, the whole-cell patch clamp recording technique was used to determine what effect NT-3 and BDNF had on the function and expression of glutamate, GABA and glycine receptors on postnatal SGN. Receptor currents induced by the agonist of each receptor were recorded from SGN cultured with or without BDNF or NT-3. NT-3 and BDNF exerted different effects. NT-3, and to a lesser extent BDNF, enhanced the expression of GABA receptors and had comparatively little effect on glutamate receptors. Absence of BDNF and NT-3 resulted in the emergence of glycine-induced currents; however, glycine receptor currents were absent from the short term cultured SGN. In contrast, NT-3 and BDNF suppressed glycine receptor expression on SGN. These results indicate that NT-3 and BDNF exert a profound effect on the types of neurotransmitter receptors expressed on postnatal SGN, results that may have important implications for neural development and plasticity.
Keywords: Spiral ganglion, Glycine, BDNF, NT-3, Plasticity, Whole-cell recording
INTRODUCTION
Excitatory and inhibitory neurotransmitter receptors play key roles in regulating the function and plasticity of the central and peripheral nervous system (Autere et al., 1999, Legendre et al., 2002). Glutamate, γ-amino-butyric acid (GABA) and glycine receptors (GlyR) are known to undergo significant functional changes during critical stages of development both in vivo and in vitro (Pisani et al., 1997, DeLorenzo et al., 1998, Kotak et al., 2001, Legendre et al., 2002). In the central auditory system, there is a profound switch from GABAergic to glycinergic neurotransmission in the lateral superior olivary complex (LSO) during development (Kotak et al., 1998). Interestingly, the plasticity of this inhibitory synapse is thought to be mediated by brain derived neurotrophic factor (BDNF) and neurotrophic factor-3 (NT-3), two of the most broadly expressed neurotrophic factors that bind to tyrosine kinase (Trk) receptors (Kotak et al., 2001). The developmental transition from GABAergic to glycinergic neurotransmission in the SOC was thought to occur because of the need for faster inhibitory circuits to faithfully encode small interaural time differences required for sound localization in mature animals.
Both BDNF and NT-3 are expressed in cochlea and play crucial roles in the development and survival of spiral ganglion neurons (SGN). The distribution of NT-3 and BDNF varies along a base-to-apex gradient in the cochlea (Fritzsch et al., 1997). At the embryonic stage, NT-3 knockout mice show a complete loss of SGN in the basal turn of the cochlea, but only a partial loss in middle and apical turns. In addition, the peripheral fibers of SGN shift from a normal radial pattern to one in which the surviving fibers spiral towards inner hair cells (IHC) and outer hair cells (OHC) (Fritzsch et al., 1997). However, the distribution of the NT-3 changes significantly during development (Adamson et al., 2002, Sugawara et al., 2007). After postnatal day 0, the expression of NT-3 decreases from the apical to the basal cochlear turn and this pattern persists into adulthood (Sugawara et al., 2007). In adult mice, BDNF gene expression in the vestibular system is much higher than in the cochlea (Stankovic and Corfas, 2003). Hair cells and supporting cells are major sources of NT-3 and other neurotrophic factors in the cochlea and hair cell loss purportedly leads to neurite retraction and eventual degeneration of SGN (Nadol, 1997). Chinchillas treated with high doses of gentamicin plus ethacrynic acid show massive loss of IHC and OHC throughout the cochlea a few days post-treatment; SGN begin to degenerate approximately15 days post-treatment and significant SGN loss is evident 3-4 months later (McFadden et al., 2002). Infusion of NT-3, BDNF or the combination into damaged ears significantly enhances SGN survival and promotes neurite outgrowth (Staecker et al., 1996b, Altschuler et al., 1999). Transfection of the cochlea with a herpes virus expressing BDNF significantly enhanced SGN survival (Miller et al., 1997). BDNF and NT-3 promote the survival of SGN in culture and protect SGN from a variety of ototoxic drugs (Zheng et al., 1995, Staecker et al., 1996a, Gao and van den Pol, 1999, Lalwani et al., 2002, McGuinness and Shepherd, 2005)
Neurotrophins have a profound effect on the functional properties of SGN. In vivo application of NT-3 beneath the IHC caused a rapid increase in NMDA and AMPA induced firing of SGN neurons (Oestreicher et al., 2000); K252a, a Trk receptor antagonist, blocked this effect. NT-3 and BDNF have a profound effect on the adaptation properties of cultured SGN. In vitro, application of BDNF to apical turn SGN caused neurons to switch their discharge patterns from slow to fast adapting in response to electrical stimulation whereas application of NT-3 caused neurons to shift towards slow adapting responses (Adamson et al., 2002). In contrast, application of NT-3 to basal turn SGN caused spike adaptation patterns to switch from fast to slow; however, BDNF had no effect on basal turn SGN. The NT-3/BDNF induced shifts in SGN adaptation patterns appear to be mediated by the Kv.1 potassium channel (Adamson et al., 2002, Mo et al., 2002) since the selective Kv1.1 potassium channel blockers DTX-K and α-DTX shifted rapidly adapting SGN to slow adapting.
Neurotrophins not only influence the expression of voltage gated ion channels, but also regulate the expression of inhibitory and excitatory neurotransmitter receptors. In some cases, BDNF increases the expression of dopamine D3 and GABA-A receptors while in other cases it decreases GABA-A receptor expression (Brunig et al., 2001, Yamada et al., 2002, Guillin et al., 2003). NT-3 also alters the expression of NMDA, dopamine and GABA receptors (Hyman et al., 1994, Sah et al., 1997). While BDNF and NT-3 have been found to alter the expression of voltage and ligand gated receptors in several brain regions, little is known about their effects on SGN. To address these issues, we monitored the expression of glutamate, GABA, and GlyRs in murine, postnatal SGN using the whole cell patch clamp technique and immunocytochemistry.
METHODS
Spiral ganglion cultures
Approximately 50 C57BL/10J mice (The Jackson Laboratory) between postnatal day 0 and 5 (P0-P5) were used in these studies. Mice were killed by cervical decapitation and the cochleas quickly removed and placed in cold Hanks balanced salt solution (HBSS) (GIBCO). The cochlear capsule was opened, the cochlear lateral wall and the spiral lamina were removed, and the basilar membrane (BM) containing the organ of Corti and SGN were placed in HBSS containing 0.25% EDTA-trypsin at 37 °C for 20 min. Trypsin was inactivated by adding 10% fetal calf serum (GIBCO) to the solution. The solution was centrifuged at 250 g for 5 min, the supernatant removed, the pellet re-suspended in culture medium and gently triturated. The solution containing the dissociated SGN was plated onto negatively charged 35 mm culture dishes (BD Falcon, Becton-Dickson). Isolated SGN were maintained in culture medium which contained 90% DMEM, 10% fetal calf serum and 100 units/ml of penicillin. The culture dishes were covered and placed in a humidified CO2 (5%) incubator (Thermo Forma, Model 3110) at 37 °C. In some cases, BDNF (5 ng/ml, B-3795, Sigma) or NT-3 (5 ng/ml, N-1905, Sigma) was added to the culture medium. Cultures were maintained for 2-4 weeks and the culture medium was changed every two days.
Immunocytochemistry
Cochlea cultures were fixed with 4% paraformaldehyde in 0.1 M sodium phosphate buffer (pH 7.0) for 20 min at room temperature and then washed three times for 5 min with phosphate buffered saline (PBS, 1X, 0.01 M, pH 7.0) at room temperature. The cell membranes were permeabilized with 0.1% Triton X-100 in PBS for 5 min. Cells were washed three times for 5 min with PBS (1X, 0.01 M) at room temperature. Afterwards, specimens were immersed for 1-2 h at room temperature in a blocking solution containing 5% normal goat serum (NGS) in PBS. Specimens were treated with a primary antibody in PBS containing 5% NGS and incubated overnight in primary antibody at 4 °C. All of the specimens were labeled with a monoclonal antibody against neurofilament 200 kDa (NF-200, N1042, Sigma) plus a polyclonal antibody against GlyR α1 and α2 subunits (rabbit; AB5052, Chemicon), GABA-A receptors (rabbit, AB5563, Chemicon) or glutamate receptor 2 (polyclonal, rabbit, AB1768, Chemicon). Afterwards, the tissues were washed three times with PBS (1X) for 15 min and then treated with a secondary antibody from the appropriate species in blocking solution (5% NGS) for more than 1 h at room temperature.
Secondary antibodies were Alexa 555 anti-rabbit IgG antibody (1:200-1:2000, Molecular Probes, A-21428) and Alexa 488 anti-mouse IgG antibody (1:200-1:2000, Molecular Probes, A-11001). Samples were rinsed three times in 0.1 M PBS, mounted in glycerin on glass slides, cover slipped, examined under a fluorescence microscope (Zeiss, Axioskop 50) and photographed with a digital camera (Nikon 922). Filter setting suitable for imaging Alexa 555 (excitation 568 nm, emission 580 nm) and Alexa 488 (excitation 488 nm, emission 520 nm) were used to examine the samples. Photomicrographs were stored on a personal computer and processed with Adobe Photoshop (Adobe Systems, San Jose, California). Each staining was performed at least times for a given condition.
Whole cell patch clamp recordings
Glass electrodes were pulled from thin wall glass tubing (Drummond Scientific Co., 1.2 mm outer diameter) using a pipette puller (PC-84, Sachs-Flaming Micropipette Puller). Electrodes were fire-polished on a microforge (MF-830, Narishige) before use. Electrode tip diameter was approximately 1-2 microns and the resistance was typically 1-3 MΩ measured in HBSS when the electrode was filled with 140 mM KCl. HEPES buffered HBSS solution was used as the external recording solution for measuring sodium, potassium and neurotransmitter receptor currents. The HBSS contained, in mM: NaCl 137, Na2HPO4 0.2, KCl 5.4, KH2PO4 0.4, MgSO2 0.8, CaCl2 1.3, glucose 5.6 and HEPES 10. The pipette used to record Na+ and K+ currents contained, in mM: KCl 120, KF 20, NaCl 2, MgCl2·2H2O 2, EGTA 10 and HEPES 10. For all solutions, pH was buffered to 7.3 with KOH and the osmolarity (Micro Osmometer, Precision Systems) adjusted with sucrose to 290 mOsm.
Whole cell patch clamp recordings were carried out at room temperature (~20 °C) in cell culture dishes. Prior to patch clamp recording, the culture medium in the dish was replaced with HEPES buffered HBSS and the dish was mounted on an Olympus inverted microscope (IMT-2) using a customized holding chamber. SGN were visualized using a phase contrast objective. Images were captured by a video camera (WPI OS 40D) attached to the microscope; the output of the camera was displayed on a video monitor (Javelin BWM12) and digitized to the computer through a video card.
The recording glass electrode was back filled with recording solution and installed on the head stage of the patch-clamp amplifier (Axon 700A, Molecular Devices) mounted on a hydraulic micromanipulator (Narishige, MC-35A). The target SGN was identified under the microscope and the electrode was gradually pressed on to the soma of the cell while negative pressure was applied through the electrode thereby drawing the cell’s membrane into the tip of the pipette. The resistance through the pipette in the cell attached mode was monitored using patch clamp software (Axon Inst., pCLAMP 8.01). Negative pressure was increased until the tight seal was ≥ 1 GΩ. The series capacitance was compensated using the amplifier and software; then a brief electric shock was applied to break the cell’s membrane and establish the whole cell, tight seal (>1 GΩ) recording configuration.
The whole cell current response was recorded in voltage-clamp mode and the voltage response was recorded in current-clamp mode (holding potential -70 mV unless otherwise noted). Receptor currents were recorded under voltage-clamp while applying various neurotransmitter receptor agonists or antagonists on to the cell using a puffer electrode positioned close to the SGN soma. Unless otherwise noted glutamate, glycine and GABA receptor agonists and antagonists were all purchased from Sigma. Alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), an AMPA receptor agonist, kainic acid (KA), a kainic/AMPA receptor agonist and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), a competitive AMPA/KA receptor antagonist, were used to test glutamate receptors. Glycine and strychnine were used to test GlyRs. GABA and bicuculline, a GABA-A receptor antagonist, were used to test GABA-A receptors. Ion and receptor channel blockers were applied alone or together through a puffer electrode (100 μm) connected to a DAD-12 superfusion system (ALA Inst.). Whole cell currents and voltage responses were amplified, digitized (DigiData 1200, Axon Inst.) and analyzed by pCLAMP software (8.01 Axon Instruments). Drug application through the DAD-12 system was under software control and could be triggered by the patch clamp software. Statistical analyses were carried out with SigmaStat 2.03 (Systat) or GraphPad Prism 5.01. Results are presented as mean ± 1 standard deviation (SD) unless otherwise noted.
This research was approved by the Institutional Animal Care and Use Committee at University at Buffalo.
RESULTS
Firing properties
The resting potential and the action potential (AP) were measured when the whole cell recording condition was established in SGNs cultured in neurotrophin free medium (untreated) for different durations. The mean resting potential of SGN maintained in culture from 1 day (C1) to 28 days (C28) was −54 ± 6.5 mV (n = 53). The mean resting potentials of SGN maintained in culture for C1-C3, C4-C6, C7-C13 and C14-C28 were -53.1 ± 10 (n = 5), -50.1 ± 8.8 (n =15), -56.4 ± 7.2 (n = 18) and -51.5 ± 6.5 (n = 9) respectively. The resting potentials did not differ significantly across groups (One-way ANOVA, P>0.05).
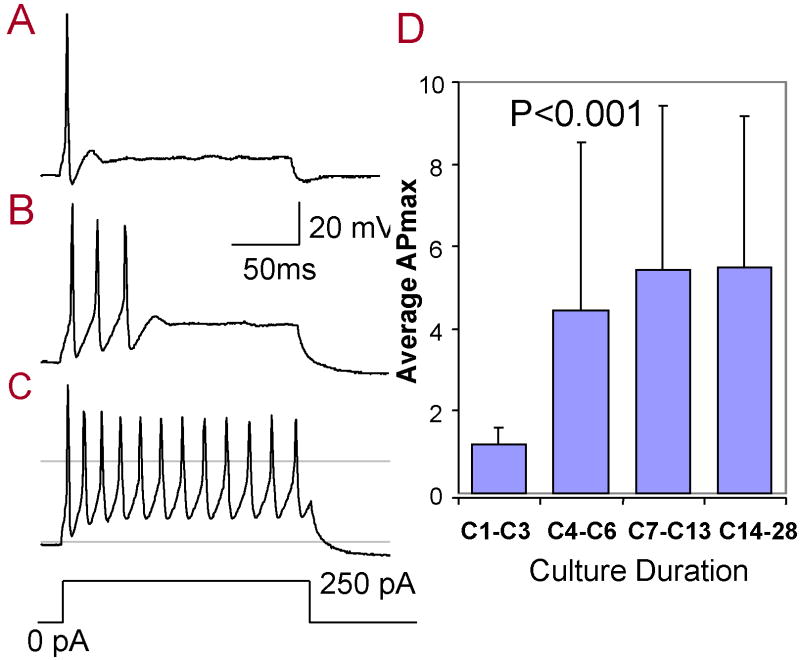
APs were also recorded from SGN in current-clamp by applying current steps from −600 to 250 pA (50 pA steps, 140 ms duration). Figure 1A showed a typical response recorded under current-clamp from a SGN maintained in culture for 1 day. A single AP was evoked by a suprathreshold current stimulus (0 to 250 pA, 140 ms). Most (31 of 35, 89%) SGN maintained in culture from C1-C3 produced only a single AP to the current step. However, multiple APs (>3) were evoked from SGN cultured long-term. As illustrated in Figure 1B-C, 3 spikes were evoked in the SGN cultured to C7 and 12 spikes were induced at C14. Multiple spikes were recorded from 67% of SGN (28 of 42) maintained in culture from C7 to C28. The average number of APs evoked by a suprathreshold current step was only 1.2 ± 0.4 (n = 11) at C1-C3 and increased to 4.4 (± 4.1, n = 9), 5.4 (± 4.0, n = 19), 5.5 (± 3.7, n = 10) at C4-C6, C7-C13, C14-C28 respectively. The mean number of evoked APs varied significantly across time (One-way ANOVA, P = 0.02, F(3, 45) = 3.64); a post-hoc analysis showed a significant difference between C1-C3 and C7-C13, C1-C3 and C14-C28 (Tukey’s Multiple Comparison Test, P < 0.05).
Figure 1.
Action potentials recorded from SGN in response to a 140 ms, 250 pA current step. Typical action potential firing patterns elicited from SGN cultured one day (C1), seven days (C7) and 28 days (C28). (C) The average of the maximum number of action potentials (APmax) increased significantly with culture duration (One-way ANOVA, P = 0.02, F(3, 45) = 3.64).
Glycine-induced receptor currents
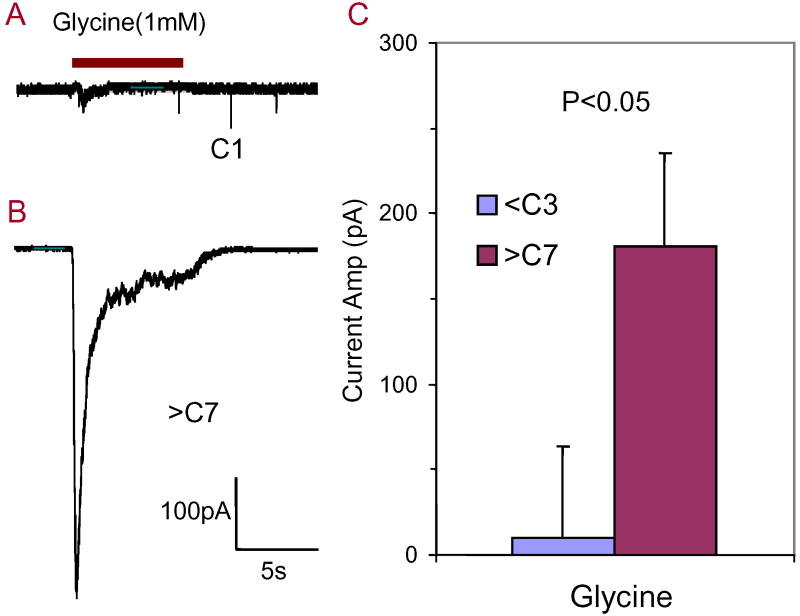
To determine if GlyRs are constitutively expressed on postnatal SGN or if neurotrophins could induce expression, whole cell recordings were obtained from SGN at a holding potential of -70 mV while 1 mM glycine was applied through a puffer electrode. When glycine (1 mM) was applied to C1-C3 SGN cultures, glycine produced an extremely weak response in 3 of 16 neurons (Figure 2A) or had no effect in 13 of 16 cells. However, a robust glycine-induced inward current was recorded from all SGN (8 of 8) cultured in neurotrophin-free medium from C7 to C28 (Figure 2B). The glycine-induced current rapidly desensitized consistent with previous reports (Sergeeva and Haas, 2001, Tao and Ye, 2002). The average amplitude of the glycine-induced current (1 mM) was -181 ± 150 pA (n = 8) from C7-C28. The average amplitude of the glycine induced current obtained from C7-C28 SGN was significantly larger than that recorded from SGN cultured C1-C3 (Figure 2C, n = 5) (Student’s t-test, P = 0.03).
Figure 2.
Developmental changes in glycine-induced currents. (A) Extremely small glycine-induced current present in SGN maintained in culture 1 day (C1). (B) Typical large amplitude, rapidly adapting glycine-induced current obtained from SGN maintained in culture for more than 7 days (>C7). (C) Mean amplitude of glycine-induced currents recorded from SGN cultured for more than 7 days (>C7) was significantly greater than from SGN cultured for less than 3 days (<C3) (Student’s t-test, P = 0.03).
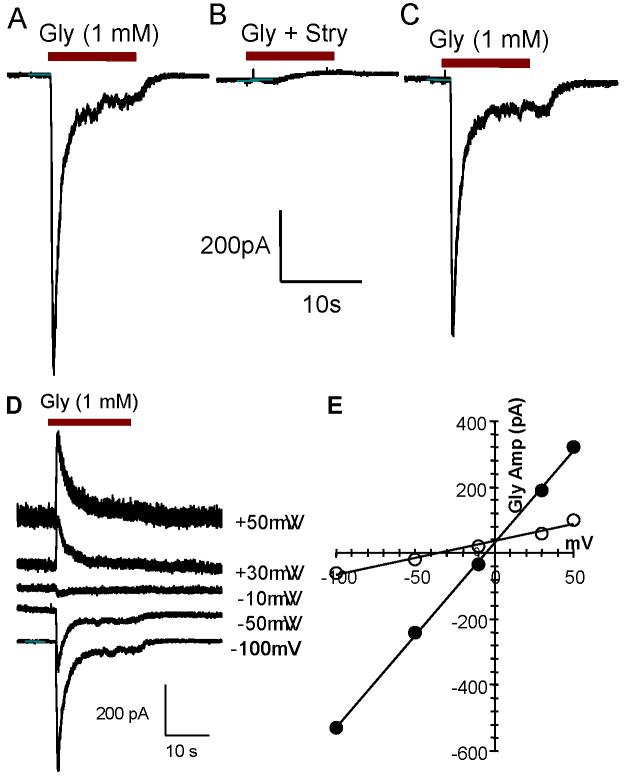
Glycine-induced (1 mM) inward currents (Figure 3A) were totally blocked by 10 μM strychnine (Figure 3B), a GlyR antagonist, and recovered 2-3 minutes after strychnine was washed out (Figure 3C). The glycine induced current was tested under different holding potentials with different chloride concentrations in the pipette (Figure 3D). With 140 mM of chloride in the pipette solution, the amplitude of the inward current (negative) decreased when the holding potential increased from -100 mV to -10 mV. The polarity of the glycine-induced current switched from inward (negative) to outward (positive) when the holding potential changed from -10 mV to +30 mV (Figure 3D). The reversal potential was close to 0 mV (Figure 3E, solid circles); this is near the expected reversal potential predicted by the Nernst equation when the chloride concentration in the pipette and bath solution are equal (equilibrium potential = 20 ln ([Cl-]in / [Cl-]out)). When the chloride concentration in the pipette was changed to 20 mM, the reversal potential was approximately -40 mV consistent with the predicted reversal potential of -39 mV (Figure 3E, open circles). These results suggest the glycine-induced current is mainly carried by chloride consistent with previous reports (Kotak et al., 1998, Kraushaar and Backus, 2002).
Figure 3.
Glycine-induced currents recorded from SGN cultured more than 7 days. Recordings obtained in voltage-clamp mode from a holding potential of -70 mV; 1 mM glycine puffed on to cell in all panels. (A) Large amplitude glycine-induced current rapidly inactivates during glycine administration. (B) Glycine-induced current totally blocked by strychnine (0.1 mM), a glycine receptor antagonist. (C) Glycine-induced current recovered after strychnine was washed out. (D) Amplitude of negative (inward) glycine-induced current decreased as the holding potential changed from -100 mV to -10 mV; glycine-induced current become positive (outward) between -10 mV and +30 mV. (E) I/V curve of glycine-induced current. Reversal potential of glycine-induced current near 0 mV with similar concentration of chloride in the recording pipette (140 mM) and bath solution (solid circles). Reversal potential was near -40 mV when the chloride concentration in the pipette increased to 20 mM (open circles).
GlyR Immunocytochemistry
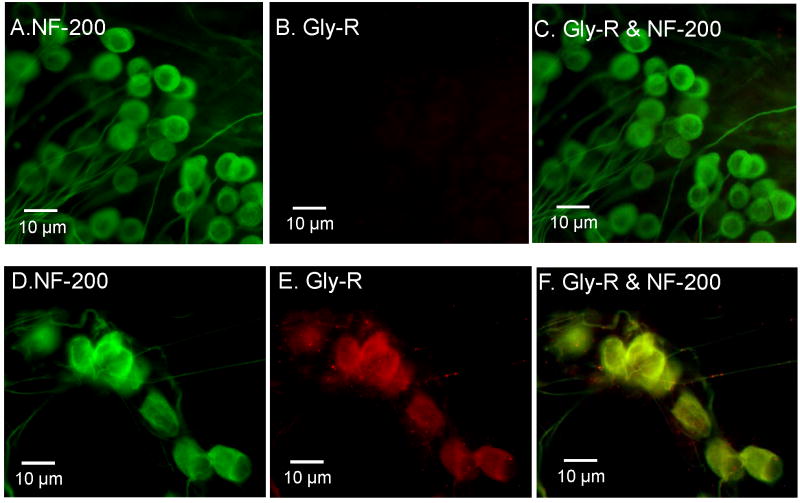
To confirm that GlyRs were present on SGN, immunocytochemistry was performed on SGN maintained in culture for C2 or from C7-28 using an antibody targeting GlyR α1 and α2 subunits. Figure 4A-C shows the typical immunostaining of SGN cultured for C2 and Figure 4D-E shows the immunostaining for SGN cultured for C10. Most of the SGN cultured for C2 showed strong NF-200 immunolabeling (Figure 4A, C), but no evidence of GlyR immunolabeling (Figure 4B, n = 10) consistent with the physiological results (Figure 2A). In contrast, strong GlyR immunolabeling was present on some SGN maintained in culture for C10 as well as immunolabeling for NF-200 kD (Figure 4D, n = 3). Figure 4F, a merger of Figure 4D and 4E, shows yellow labeling from the overlap of GlyR and NF-200 kD immunolabeling. The C10 results are consistent with the physiological results showing the presence of glycine-induce currents in long term SGN cultures.
Figure 4.
Immunostaining using a polyclonal antibody against glycine receptors (red) and a monoclonal antibody against neurofilament NF-200 kD (green) expressed on SGN cultured for 2 days (A-C) or 10 days (D-F) in neurotrophin free medium. (A) NF-200 kD, a neuron specific marker, expressed on SGN cultured for 2 days (C2). (B) Glycine-receptor immunolabeling absent from SGN at C2. (C) Merged image of A and B only shows NF-200 kD immunolabeling. (D) NF-200 kD (green) expressed on SGN maintained in culture for 10 days (C10). (E) Glycine receptor immunolabeling (red) expressed on SGN at C10. (F) Merged image of D and E showing co-expression (yellow) of NF-200 (green) and glycine receptors (red) on SGN at C10.
Effect of NT-3 and BDNF on glycine-induced currents
Neurotrophic factors can modulate the expression and function of neurotransmitter receptors (Heese et al., 2000, Brunig et al., 2001, Xiong et al., 2002). Since NT-3 and BDNF play important roles in the development and survival of SGN, NT-3 (5 ng/ml) and BDNF (5 ng/ml) were applied to SGN cultures to evaluate their effects. The mean resting potential of SGN maintained under neurotrophin-free culture conditions for C7-14 was -50 ± 12 mV (n = 12); the mean resting potential was -54 ± 6 mV (n = 28) when cultured with BDNF (5 ng/ml) and -51 ± 6 mV (n = 5) when cultured with NT-3. The mean resting potentials of SGN maintained in neurotrophin-free medium, BDNF or NT-3 were not significantly different from one another (One-way ANOVA, p > 0.05).
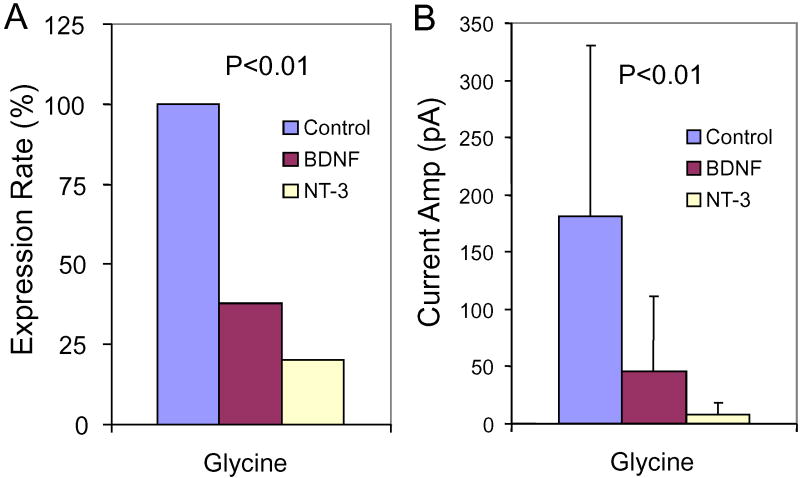
Figure 5A shows the percentage of SGN expressing glycine-induced currents. All (8 of 8) SGN maintained in neurotrophin-free medium from C7-14 expressed glycine-induced currents. In contrast, only 1 of 6 (17%) SGN cultured with NT-3 and only 14 of 37 (38%) cultured with BDNF expressed glycine-induced currents. The proportions of SGN expressing glycine-induced currents in neurotrophin-free medium or medium containing BDNF or NT-3 were significantly different (p = 0.002, Chi-square test).
Figure 5.
SGN cultured from C7-C14 in neurotrophin free medium or medium containing 5 ng/ml of BDNF or NT-3. (A) Percentage of SGN expressing glycine-induced currents significantly reduced (Chi-square test, p = 0.002) in BDNF or NT-3 treated cultures relative to control. (B) Mean amplitude (± SD) of glycine-induced currents recorded from SGN cultured in neurotrophin free medium was significantly (One-way ANOVA, P = 0.003, F(2, 25) = 7.45) greater than in SGN cultured with BDNF or NT-3. A post-hoc analysis showed a significant difference between Control vs NT-3 and Control vs BDNF groups (Tukey’s Multiple Comparison Test, P < 0.05).
The mean amplitudes of glycine-induced currents were -46 ± 65 pA (n = 14) in SGN (C7-C14) cultured with BDNF, -8 ± 17 pA (n = 6) in SGN (C7-C14) cultured with NT-3, and -181 ± 150 pA (n = 8) in SGN cultured with neurotrophin-free medium (Figure 5B). The mean amplitude of the glycine-induced current was significantly greater in SGN cultured in neurotrophin-free medium than from SGN cultured in BDNF or NT-3 (One-way ANOVA, P = 0.003, F(2, 25) = 7.45). A post-hoc analysis showed a significant difference between neurotrophin free vs. NT-3 and neurotrophin free vs. BDNF groups (Tukey’s Multiple Comparison Test, P < 0.05).
NMDA and Non-NMDA Glutamate Receptors
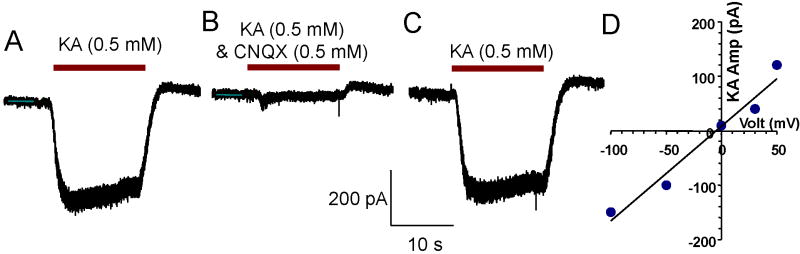
Non-NMDA receptor function in SGN was evaluated using AMPA and KA receptor agonists and antagonists. KA-induced currents were recorded from SGN (n = 49) between C1-14 in neurotrophin free medium. No significant development changes were observed during this period. KA (0.5 mM) or AMPA (0.5 mM) induced a negative (inward) current in most SGN cultured from C1-14 (45 of 49, holding potential −70 mV). Figure 6A shows typical KA-induced (0.5 mM) inward currents recorded from a SGN maintained in neurotrophin free culture medium until C10. The KA-induced current consisted of a fast onset followed by sustained activation and returned to baseline when KA was washed out. The KA-induced current was reversibly blocked by CNQX (0.1 mM), a non-NMDA receptor antagonist, consistent with previous reports (Ruel et al., 1999) (Figure 6B). The KA-induced current quickly recovered after CNQX was washed out (Figure 6C). The KA-induced current was inward at negative holding potentials and outward at positive potentials (Figure 6D). The I/V curve for the KA-induced current was linear and reversed polarity around 0 mV These results suggest that the KA-induced currents were carried by a non-selective cation channel consistent with glutamate receptors (Johnston and Wu, 1999).
Figure 6.
(A) KA (0.5 mM) induced inward currents recorded from a typical SGN that was cultured neurotrophin free medium for C10. Holding potential is -70 mV. KA-induced current consisted of fast onset, sustained response and rapid decay after KA was washed out. (B) KA-induced current was blocked by CNQX (0.1 mM), a non-NMDA receptor antagonist. (C) KA induced current quickly recovered after CNQX was washed out. (D) I/V curve for KA-induced current linearly related to voltage with a reversal potential near 0 mV. KA-induced current switched from inward at negative holding potentials to outward at positive holding potentials.
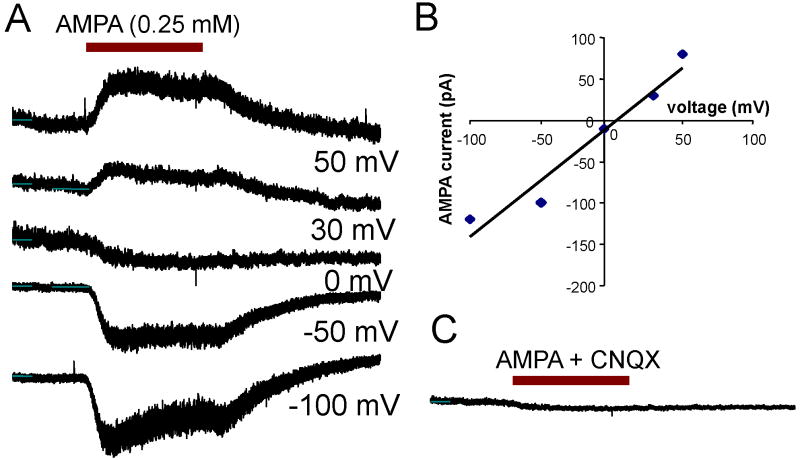
AMPA-induced currents were also recorded from SGN (n = 6) between C1-14 in neurotrophin free medium. No significant development changes were observed during this period. Figure 7A shows a series of AMPA-induced (0.5 mM) currents obtained from a typical SGN at holding potentials from -100 to +50 mV. At -100 mV, an inward current was recorded with a rapid onset followed by a slow, partial decay. The amplitude of the AMPA-induced current decreased as the holding potential approached 0 mV. As the holding potentials increased from 0 mV to +50 mV, the polarity switched from negative to positive and current amplitude increased. A typical I/V curve of the AMPA-induced current is plotted in Figure 7B. The reversal potential was close to 0 mV, and the I/V curve exhibited a fairly linear relationship from -60 to +50 mV (Figure 7B). These results suggest that the AMPA-induced current is carried by non-selected cation consistent with previous reports (Johnston and Wu, 1999). The AMPA-induced current was totally blocked by 0.1 mM CNQX (Figure 7C), an AMPA receptor antagonist and quickly recovered after CNQX was washed out (Data not shown).
Figure 7.
(A) AMPA (0.5 mM) induced currents recorded from SGN. Amplitude of AMPA-induced inward (negative) current decreased from -100 mV to 0 mV and reversed polarity (outward current) at positive potentials. (B) I/V curve of AMPA-induced currents nearly linear with a reversal potential near 0 mV (cation concentration of pipette solution similar to bath solution). (C) AMPA-induced current blocked by CNQX (0.1 mM), a non-NMDA receptor antagonist.
Attempts were made to record NMDA-induced currents from 12 SGN maintained from C1-C14 in neurotrophin free medium. NMDA failed to induce a measureable current in SGN (0 of 2) (data not shown). These results suggest that NMDA receptors are either absent or inactivate during normal culture conditions consistent with previous reports (Ruel et al., 1999, Peng et al., 2003).
Effect of BDNF and NT-3 on KA-Induced Currents
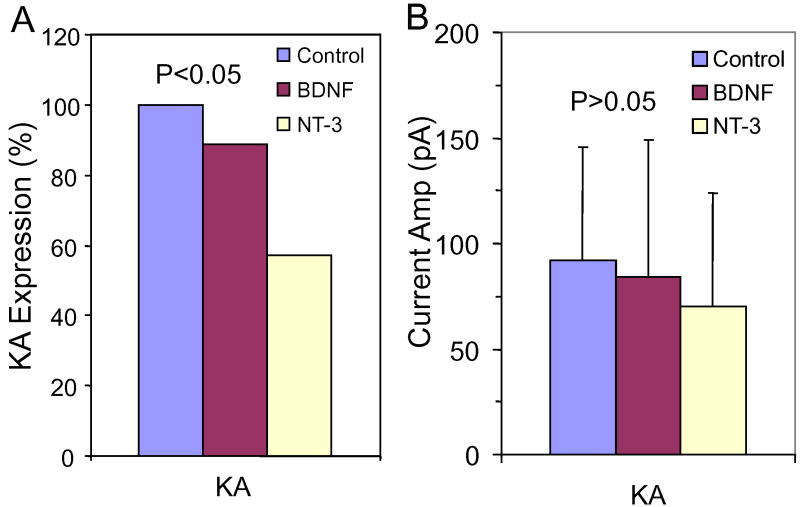
To determine if neurotrophins can modulate the expression of glutamate receptors on SGN, BDNF or NT-3 was added to the culture medium. KA-induced (0.5 mM) currents were recorded from all SGN maintained in neurotrophin free medium from C7-C14 (8/8) consistent with previous results. When BDNF (5 ng/ml) was added to the culture medium, the percentage of SGN expressing KA-induced currents fell to 88% (31/35). However, when NT-3 (5 ng/ml) was added to the culture medium, only 4 out of 7 SGN (57%) expressed KA-induced currents (Figure 8A). The proportions of SGN expressing KA-induced currents in neurotrophin-free medium or medium containing BDNF or NT-3 were significantly different (p = 0.04, Chi-square test). The mean amplitudes of KA induced currents recorded from SGN were -92 ± 54 pA (n = 8) in neurotrophin free medium, -84 ± 46 pA (n = 31) in BDNF and -70 ± 26 pA (n = 4) in NT-3 (Figure 8B) (Not significant).
Figure 8.
SGN cultured from C7-C14 in neurotrophin free medium or medium containing 5 ng/ml of BDNF or NT-3. (A) Percentage of SGN expressing KA-induced currents slightly lower in BDNF and NT-3 treated cultures than in neurotrophin free controls (Chi-square test, P = 0.04). (B) Mean amplitude (± SD) of KA-induced currents recorded from SGN cultured in neurotrophin free medium was not significantly (NS) different from SGN cultured with BDNF or NT-3.
AMPA Receptor Immunolabeling
Figure 9 shows examples of AMPA receptor and NF-200 kD immunostaining on SGN maintained in neurotrophin free medium from C7 and C14 (n = 5). Strong NF-200 kD immunolabeling was observed on the soma and neurites of most SGN at C7 (Figure 9A) and C14 (Figure 9D). AMPA receptor immunolabeling was present on some SGN, but less intense than NF-200 kD immunolabeling at C7 and C14 (Figure 9B-D). The merger of AMPA receptor and NF200 kD immunolabeling is shown in Figures 9C and 9F. The expression of AMPA receptor immunolabeling on SGN from C7 and C14 is consistent with the physiological results.
Figure 9.
AMPA receptor (red) and NF-200 kD (green) immunolabeling of SGN at C7 (A-C) and C14 (D-F). (A) Strong NF-200 kD staining on SGN at C7. (B) Weak AMPA receptor staining on SGN at C7 (arrows). (C) Merger of figure (A) and (B) shows the co-expression of NF-200 kD and AMPA receptors. (D) Strong staining of NF-200 kD on SGN at C14. (E) Most SGN stained with AMPA receptors at C14 (arrows). (F) Merger of (D) and (E) shows co-expression of NF-200 and AMPA receptors.
GABA receptor expression
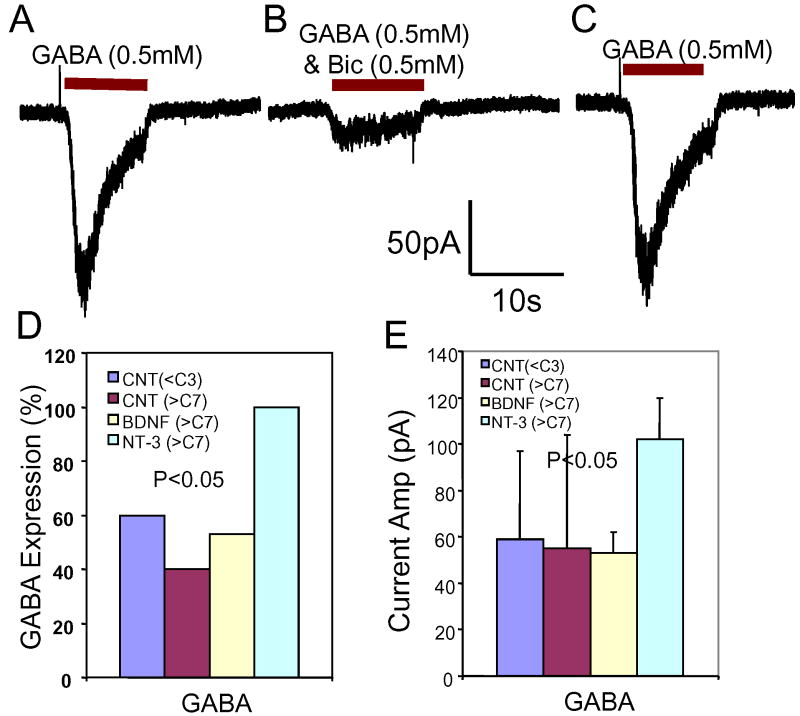
Previous studies indicate that glutamate-induced excitation of SGN can be modulated by GABA (Lin et al., 2000). Developmental changes in GABA-A receptor expression was assessed by recording GABA-induced currents from SGN cultured with or without NT-3 or BDNF. Figure 10A shows a typical GABA-induced (0.5 mM) inward current recorded from a SGN at C7. GABA induced a large inward current with a fast onset followed by a rapid, partial decay. The GABA-induced current disappeared when GABA perfusion ended. The GABA-induced current was largely blocked by 0.5 mM bicuculline, a GABA-A receptor antagonist (Figure 10B). The GABA-induced current recovered quickly when bicuculline was washed out (Figure 10C). These results suggest that the GABA-induced current expressed on SGN is mainly carried by the GABA-A receptor subtype.
Figure 10.
GABA (0.5 mM) induced inward current recorded from SGN (holding potential -70 mV). (A) GABA-induced current at C7 has a fast onset followed by a slower decay after 1-2 seconds. (B) GABA induced current blocked by bicuculline (BIC, 0.5 mM), a GABA-A receptor antagonist. (C) GABA induced current quickly recovered after BIC was washed out. (D) In the control neurotrophin free cultures (CNT), GABA-induced currents were recorded from 60% of short term SGN cultures (<C3) and 40% of long term SGN cultures (>C7). Adding NT-3 to the cultures increased the number of GABA positive SGNs to nearly 100%; adding BDNF only slightly increased the percentage of GABA-positive SGN. (E) Amplitude of GABA-induced currents recorded from long term SGN cultured in NT-3 was greater than in those recorded from SGN cultured with BDNF or neurotrophin free medium.
To investigate the developmental changes in GABA receptor expression, GABA receptor currents were assessed in SGN cultured short term (C1 to C3) or long term (C7 to C14) without or with BDNF or NT-3. GABA-induced (1 mM) currents were recorded from 6 out of 10 SGN (60%) cultured short term in neurotrophin free medium. The mean amplitude of the GABA-induced current was -59 ± 38 pA (n = 6) (Figure 10D). The percentage of SGN with GABA-induced currents dropped to 40% (4 out of 10) in neurotrophin-free, long term cultures. The mean amplitude of the GABA-induced (1 mM) current was -55 ± 49 pA (n = 4). When SGN were cultured in NT-3 from C7 to C14, GABA-induced currents were recorded from 7 out of 7 SGN (100%); the mean amplitude was -101 ± 18 pA (n = 7). When SGN were cultured in BDNF from C7 to C14, GABA-induced currents were recorded in 9 out of 17 SGN (53%) with a mean amplitude of -53 ± 9 pA (n = 9). The percentage of GABA-positive SGN was significantly different when cultured in NT-3, BDNF or neurotrophin free medium (P = 0.04, Chi-square test). The results indicate that NT-3 is mainly responsible for the increased expression of GABA-A receptors. The amplitudes of GABA-induced currents were significantly greater in SGN cultured with NT-3 than in neurotrophin free medium or BDNF (One-way ANOVA, p < 0.05). These data suggest NT-3 enhances the amplitude of GABA-induced current in SGN.
GABA receptor immunolabeling
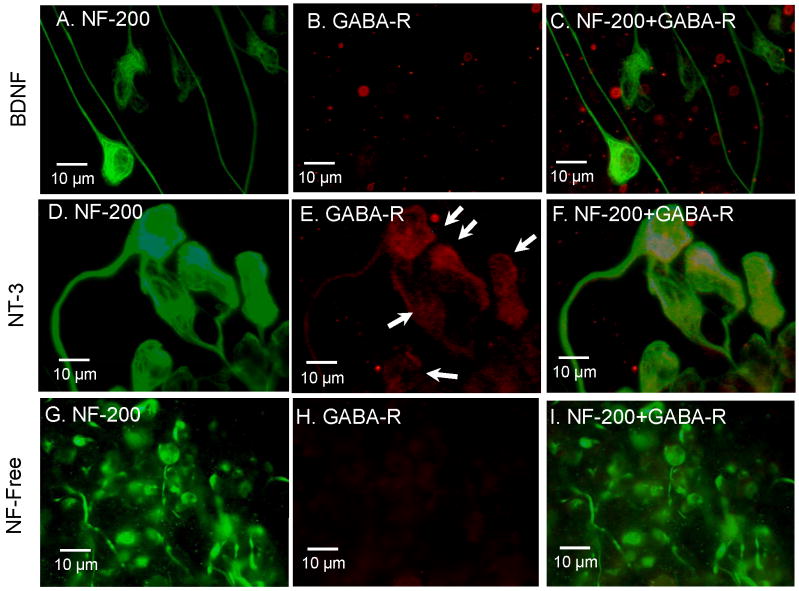
SGN show strong NF-200 kD immunolabeling in most of SGN cultured in BDNF, NT-3 or neurotrophin free medium (Figure 11 A, D, E). Some SGN were immunopostive for GABA-A receptors and NF-200 kD when cultured with NT-3 (Figure 11E, F, n = 4); these results are consistent with the preceeding physiological results (Figure 10A). GABA-A receptors immunolabeling was weak or absent in SGN cultured in BDNF (Figure 11B-C, n = 5) or neurotrophin free medium (Figure 11H, n = 7).
Figure 11.
GABA receptor immunostaining of SGN cultured in medium with BDNF (A-C), NT-3 (D-F) or neurotrophin free (G-I). (A) Strong NF-200 kD immunolabeling (green) of SGN cultured in medium with BDNF. (B) Little or no GABA receptor immunolabeling (red) on SGN cultured with BDNF. (C) Merger of A and B. (D) Strong NF-200 kD immunolabeling of SGN cultured in NT-3. (E) GABA receptor immunolabeling (red) expressed on SGN cultured with NT-3. (F) Merger of figure D and E showing co-expression of NF-200 kD and GABA-R. (G) SGN stained with NF-200 kD NF-free medium. (H) No GABA receptor immunolabeling expressed on SGN cultured with NF-free medium. (I) Merger of figure G and H.
DISCUSSION
The main findings of this project were as follows: (1) When SGN were cultured from C1-C28 in the absence of NT-3 and BDNF, the AP firing pattern of SGN changed from fast adapting to slow adapting. (2) Glycine induced currents were recorded from SGN when cultured in the absence of NT-3 and BDNF for more than 7 days (C7). GlyRs were identified on SGN cultured in neurotrophin free medium. When BDNF or NT-3 was added to SGN cultures, the percentage of neurons responding to glycine and the amplitude of glycine-induced current decreased. (3) KA-induced currents were expressed in all SGN cultured in the absence of NT-3 and BDNF. The expression of KA-induced currents decreased to 90% when SGN were cultured in BDNF and declined to 50% when cultured in NT-3. These results suggest that NT-3 could affect the expression of non-NMDA receptors on SGN. (4) When SGN were cultured in the absence of NT-3 and BDNF, the percentage of SGN expressing GABA-R and the amplitude of GABA-induced currents were low. Adding NT-3 caused a large increase in the percentage of GABA-R expressing neurons and a large increase in GABA-induced currents whereas BDNF had no effect. The mechanisms underlying these developmental changes and their relevance to in vivo functions are discussed below.
AP firing patterns during development
Under neurotrophin free conditions, all short term cultured SGN (C1-C3) showed a single AP when stimulated with a supra-threshold current pulse (250 pA, 240 ms duration) whereas long term cultured SGN (> C5) generated multiple AP, i.e., they showed less adaptation. One possible explanation for the increase in multiple AP in long-term SGN cultures is that it is due to a down regulation of Kv1.1 DTX-sensitive potassium channels (Mo et al., 2002). It is unclear what effects BDNF and NT3 would have on neural adaptation and Kv1.1 DTX-sensitive potassium channels. The effects of BDNF and NT-3 could be tested by pharmacologically by applying DTX to SGN cultured for different durations in the absence or presence of NT-3 or BDNF.
Developmental change in glutamate receptor expression
Glutamate is believed to be the primary excitatory neurotransmitter released by IHC on to the afferent dendrites of SGN. Consistent with previous reports, robust KA and AMPA-induced currents were recorded from most SGN studied in P1-P5 cochlear cultures (Ruel et al., 1999). In addition, KA-induced currents were present in nearly all isolated SGN cultured from C1-C28 in the absence of NT-3 or BDNF. These results indicate that non-NMDA glutamate receptors are expressed on SGN in the absence of NT-3 or BDNF. On the other hand, when SGN were cultured in the presence of NT-3, the percentage of SGN expressing KA induced currents was greatly reduced (Figure 8A). However, NT-3 did not alter the amplitude of the KA induced current and BDNF did not affect the percentage of SGN expressing KA-induced currents or the amplitude of the KA induced current.
Previous studies using immunocytochemistry and Western blots have reported that culturing neocortical neurons with BDNF enhanced the expression of AMPA type glutamate receptors, whereas NT-3 showed no effects (Narisawa-Saito et al., 1999). Moreover, BDNF knockout mice showed reduced expression of AMPA receptor proteins. Other studies have found that BDNF regulates the expression of the AMPA receptor subunit, GluR2 (Brene et al., 2000). These results indicate that BDNF can regulate the expression of AMPA receptors and may contribute to synaptic and developmental plasticity. A recent study reported that GluR2 and GluR3 are enriched in SGN in the base of the cochlea compared to the apex which may be related to the distribution of BDNF and NT-3 in the cochlea (Flores-Otero et al., 2007).
NT-3 has also been reported to acutely alter glutamate neurotransmission by altering presynaptic function. Iontophoresis of NT-3 in the cochlea increased the spontaneous and glutamate-induced firing rate of SGN; this effect was blocked by a Trk receptor antagonist (Oestreicher et al., 2000). These results indicate that NT-3 enhances glutamate transmission in adult SGN afferent terminals. However, our results indicate that NT-3 or BDNF did not enhance KA-induced currents in neonatal SGN, but only affected the percentage of neurons expressing KA-induced currents. Other studies have shown than neurotrophins enhance glutamate synaptic transmission by activating Trk receptors and enhancing the release of glutamate from pre-synaptic neurons (Lessmann, 1998). In contrast, our results indicate that NT-3 reduces the expression of KA receptors on SGN. Other studies have reported that cortical and hippocampal neurons pretreated with NT-3 or BDNF were resistant to glutamate neurotoxicity and showed a reduced influx of calcium in response to glutamate (Cheng et al., 1994). The mechanisms underlying this effect are not clear. The present results from neonatal SGN suggest that neurotrophins might reduce the expression of glutamate receptors which would reduce the influx of calcium and therefore make neurons more resistant to glutamate excitotoxicity.
Developmental changes of GABA receptors in SGN
The percentage of SGN expressing GABA-induced currents and the magnitude of the GABA-induced currents increased significantly when SGN were cultured with NT-3. NT-3 also increased GABA-R immunolabeling (Figure 10). These results suggest that NT-3 promotes the expression of GABA receptors on SGN and enhances the amplitude of GABA-induced currents in neonatal SGNs. These results are consistent with previous studies showing that GABA-induced currents were enhanced in the presence of NT-3 (Gao and van den Pol, 1999). In contrast, we found that application of BDNF caused a slight decrease in the percentage of SGN expressing GABA-induced currents. This is consistent with previous work showing that BDNF reduced miniature inhibitory postsynaptic potentials by reducing the expression of GABA-A receptor in cultured embryonic hippocampal neurons (Brunig et al., 2001).
The mechanism by which NT-3 increases the expression of GABA-induced currents is not yet clear. However, it has been reported that neuregulin, a member of the epidermal growth factor family, induces the expression of GABA receptor subunits and ACh receptor subunits, whereas BDNF had no effect on these receptors (Rieff et al., 1999, Liu et al., 2001). Neuregulin is expressed in SGN and Schwann cells (Hansen et al., 2001). Since NT-3 enhances the survival of SGN and Schwann cells (Zheng et al., 1995, Hansen et al., 2001), it is possible that neuregulin released from these cells stimulates the production of GABA-A receptors on SGN via an autocrine or paracrine route. During development, GABA and its synthesizing enzyme, GAD, appear under the IHC; this regions is normally occupied by GABAergic efferent endings (Merchan-Perez et al., 1990) which could activate GABA-A receptors expressed on SGN. Since the efferent endings are eliminated in cultured SGN, the loss of GABAergic efferent fibers may lead to the loss of GABA-receptors and GABA induced currents in cultured SGN.
BDNF signaling through trkB receptors is necessary to establish GABAergic synapses in the developing cerebellum (Rico et al., 2002). In the present study BDNF failed to enhance the expression of GABA induced currents; however, NT-3 significantly enhanced the expression of GABA-induced currents in cultured neonatal SGN. These results are consistent with previous reports showing that NT-3 strongly upregulates GABA-induced currents in neural progenitor cells (Sah et al., 1997).
Developmental changes in GlyR current
Previous studies have failed to identify GlyRs on SGN; however, GlyRs are present on vestibular ganglion neurons (Aoki et al., 1988). A more recent paper detected the mRNA of glycine-α3 and β receptors in SGN in adult rats after the onset of hearing. These results raise the possibility that the GlyRs on SGN may serve as targets olivocochlear efferent fibers in adult rats, not in neonatal rats (Dlugaiczyk et al., 2008). This may explain why glycine-induced currents have not been detected on SGN before P7. We did not observe glycine-induced currents and GlyR immunolabeling of α1 and α2 subunits on SGN cultured from C1-C3 in neurotrophin free medium (Figure 2A, 4B). However glycine-induced currents and GlyRs were expressed on SGN maintained in neurotrophin free culture medium from C7-C28 (Figure 2B, 4D) suggesting that the expression of GlyRs increased significantly in neurotrophin-free medium. Adding NT-3 or BDNF to the culture medium decreased the percentage of neurons expressing glycine-induced currents and decreased the amplitude of glycine induced currents; these effects were most pronounced in the presence of NT-3.
It is currently unclear why GlyRs are up regulated and GABA-A receptors down regulated in SGN cultured in neurotrophin free medium. However, a similar type of GABA-to-glycine receptor switch occurs in the LSO during development (Kotak et al., 1998). Glycine normally acts as an inhibitory neurotransmitter in the peripheral and central nervous systems. Activation of GlyRs induces an influx of chloride ions from the extracellular environment which hyperpolarizes postsynaptic neurons (Hille, 1992). GABA acting through GABA-receptors also causes an influx of chloride ions which hyperpolarizes the cell. However, GlyRs have a briefer hyperpolarizing response than GABA receptors; consequently the developmental switch from GABA to glycine neurotransmission in the LSO presumably enhances the temporal response properties of LSO neurons (Yang and Pollak, 1994, Klug et al., 1995, Nabekura et al., 2004). The faster response time of GlyRs would presumably enhance the ability of neurons in LSO to process small interaural time differences that are important in sound localization (Kotak and Sanes, 2003).
The mechanisms that lead to the expression of glycine-induced currents in SGN maintained in neurotrophin free medium are unclear. Spinal cord slice cultures show an absence of glycinergic inhibition in 7-day cultures followed by a pronounced increase in glycinergic inhibitions as the cultures aged (Lu et al., 2006). In developing spinal cord neurons, GlyR activation has been shown to cause depolarization, an influx of calcium and an increase in the length and number of neurites (Tapia et al., 2000). Thus, the increase in GlyR currents in long term SGN cultures may promote neurite outgrowth in SGN. Increases in glycinergic synaptic activity also enhances the survival of motor neurons (Banks et al., 2005); it would be interesting to determine if a similar survival promoting effect of GlyR occurs in long term SGN cultures. The GlyR antibody used in this experiment is specific to the α1 and α2 subunits, not to the β subunit. As the adult form of the GlyR is the heteromeric α1 and β receptor whereas neonatal form of the GlyR is presumed to be a homopentamer of five α2 subunits (Becker et al., 1988), the increasing expression of the α1 or α2 GlyRs could be induced by an increasing expression of the immature GlyRs.
Potential implication of neurotransmitter receptor plasticity
Congenital or acquired hearing loss (e.g., noise exposure, ototoxicity, presbycusis) is almost always associated with hair cell loss. Since hair cells were proposed as a major source of neurotrophins, hair cell damage during the embryonic or postnatal period would presumably decrease the supply and distribution of neurotrophins in the cochlea which in turn may affect neurite outgrowth and the survival of SGN (Ernfors et al., 1995, Miller et al., 1997, Mou et al., 1997). The results of the present study using cultured neonatal SGN indicate that the loss of neurotrophins may also alter the expression of neurotransmitter receptors expressed on SGN. Persistent down regulation of these receptors may have important implications for therapeutic efforts to regenerate hair cells in the mammalian inner ear (Izumikawa et al., 2005, Sage et al., 2005).
Acknowledgments
This work was supported in part by grants from National Institutes of Health (R01 DC06630-01, R03 DC008685-01), Deafness Research Foundation and National Organization of Hearing Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamson CL, Reid MA, Davis RL. Opposite actions of brain-derived neurotrophic factor and neurotrophin-3 on firing features and ion channel composition of murine spiral ganglion neurons. J Neurosci. 2002;22:1385–1396. doi: 10.1523/JNEUROSCI.22-04-01385.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschuler RA, Cho Y, Ylikoski J, Pirvola U, Magal E, Miller JM. Rescue and regrowth of sensory nerves following deafferentation by neurotrophic factors. Ann N Y Acad Sci. 1999;884:305–311. doi: 10.1111/j.1749-6632.1999.tb08650.x. [DOI] [PubMed] [Google Scholar]
- Aoki E, Semba R, Keino H, Kato K, Kashiwamata S. Glycine-like immunoreactivity in the rat auditory pathway. Brain Res. 1988;442:63–71. doi: 10.1016/0006-8993(88)91432-1. [DOI] [PubMed] [Google Scholar]
- Autere AM, Lamsa K, Kaila K, Taira T. Synaptic activation of GABAA receptors induces neuronal uptake of Ca2+ in adult rat hippocampal slices. J Neurophysiol. 1999;81:811–816. doi: 10.1152/jn.1999.81.2.811. [DOI] [PubMed] [Google Scholar]
- Banks GB, Kanjhan R, Wiese S, Kneussel M, Wong LM, O’Sullivan G, Sendtner M, Bellingham MC, Betz H, Noakes PG. Glycinergic and GABAergic synaptic activity differentially regulate motoneuron survival and skeletal muscle innervation. J Neurosci. 2005;25:1249–1259. doi: 10.1523/JNEUROSCI.1786-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker CM, Hoch W, Betz H. Glycine receptor heterogeneity in rat spinal cord during postnatal development. EMBO J. 1988;7:3717–3726. doi: 10.1002/j.1460-2075.1988.tb03255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brene S, Messer C, Okado H, Hartley M, Heinemann SF, Nestler EJ. Regulation of GluR2 promoter activity by neurotrophic factors via a neuron-restrictive silencer element. Eur J Neurosci. 2000;12:1525–1533. doi: 10.1046/j.1460-9568.2000.00040.x. [DOI] [PubMed] [Google Scholar]
- Brunig I, Penschuck S, Berninger B, Benson J, Fritschy JM. BDNF reduces miniature inhibitory postsynaptic currents by rapid downregulation of GABA(A) receptor surface expression. Eur J Neurosci. 2001;13:1320–1328. doi: 10.1046/j.0953-816x.2001.01506.x. [DOI] [PubMed] [Google Scholar]
- Cheng B, Goodman Y, Begley JG, Mattson MP. Neurotrophin-4/5 protects hippocampal and cortical neurons against energy deprivation-and excitatory amino acid-induced injury. Brain Res. 1994;650:331–335. doi: 10.1016/0006-8993(94)91801-5. [DOI] [PubMed] [Google Scholar]
- DeLorenzo RJ, Pal S, Sombati S. Prolonged activation of the N-methyl-D-aspartate receptor-Ca2+ transduction pathway causes spontaneous recurrent epileptiform discharges in hippocampal neurons in culture. Proc Natl Acad Sci U S A. 1998;95:14482–14487. doi: 10.1073/pnas.95.24.14482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlugaiczyk J, Singer W, Schick B, Iro H, Becker K, Becker CM, Zimmermann U, Rohbock K, Knipper M. Expression of glycine receptors and gephyrin in the rat cochlea. Histochem Cell Biol. 2008;129:513–523. doi: 10.1007/s00418-008-0387-x. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Kucera J, Lee KF, Loring J, Jaenisch R. Studies on the physiological role of brain-derived neurotrophic factor and neurotrophin-3 in knockout mice. Int J Dev Biol. 1995;39:799–807. [PubMed] [Google Scholar]
- Flores-Otero J, Xue HZ, Davis RL. Reciprocal regulation of presynaptic and postsynaptic proteins in bipolar spiral ganglion neurons by neurotrophins. J Neurosci. 2007;27:14023–14034. doi: 10.1523/JNEUROSCI.3219-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Silos-Santiago I, Bianchi LM, Farinas I. The role of neurotrophic factors in regulating the development of inner ear innervation. [Review] [59 refs] Trends in Neurosciences. 1997;20:159–164. doi: 10.1016/s0166-2236(96)01007-7. [DOI] [PubMed] [Google Scholar]
- Gao XB, van den Pol AN. Neurotrophin-3 potentiates excitatory GABAergic synaptic transmission in cultured developing hypothalamic neurones of the rat. J Physiol. 1999;518(Pt 1):81–95. doi: 10.1111/j.1469-7793.1999.0081r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillin O, Griffon N, Bezard E, Leriche L, Diaz J, Gross C, Sokoloff P. Brain-derived neurotrophic factor controls dopamine D3 receptor expression: therapeutic implications in Parkinson’s disease. Eur J Pharmacol. 2003;480:89–95. doi: 10.1016/j.ejphar.2003.08.096. [DOI] [PubMed] [Google Scholar]
- Hansen MR, Vijapurkar U, Koland JG, Green SH. Reciprocal signaling between spiral ganglion neurons and Schwann cells involves neuregulin and neurotrophins. Hear Res. 2001;161:87–98. doi: 10.1016/s0378-5955(01)00360-4. [DOI] [PubMed] [Google Scholar]
- Heese K, Otten U, Mathivet P, Raiteri M, Marescaux C, Bernasconi R. GABA(B) receptor antagonists elevate both mRNA and protein levels of the neurotrophins nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) but not neurotrophin-3 (NT-3) in brain and spinal cord of rats. Neuropharmacology. 2000;39:449–462. doi: 10.1016/s0028-3908(99)00166-5. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic channels of excitable membrane. Sunderlang: Sinauer Associates Inc.; 1992. [Google Scholar]
- Hyman C, Juhasz M, Jackson C, Wright P, Ip NY, Lindsay RM. Overlapping and distinct actions of the neurotrophins BDNF, NT-3, and NT-4/5 on cultured dopaminergic and GABAergic neurons of the ventral mesencephalon. J Neurosci. 1994;14:335–347. doi: 10.1523/JNEUROSCI.14-01-00335.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, Brough DE, Raphael Y. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005;11:271–276. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- Johnston D, Wu SM-S. Foundations of Cellular Neurophysiolgy. the MIT Press; 1999. [Google Scholar]
- Klug A, Park TJ, Pollak GD. Glycine and GABA influence binaural processing in the inferior colliculus of the mustache bat. J Neurophysiol. 1995;74:1701–1713. doi: 10.1152/jn.1995.74.4.1701. [DOI] [PubMed] [Google Scholar]
- Kotak VC, DiMattina C, Sanes DH. GABA(B) and Trk receptor signaling mediates long-lasting inhibitory synaptic depression. J Neurophysiol. 2001;86:536–540. doi: 10.1152/jn.2001.86.1.536. [DOI] [PubMed] [Google Scholar]
- Kotak VC, Korada S, Schwartz IR, Sanes DH. A developmental shift from GABAergic to glycinergic transmission in the central auditory system. J Neurosci. 1998;18:4646–4655. doi: 10.1523/JNEUROSCI.18-12-04646.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotak VC, Sanes DH. Gain adjustment of inhibitory synapses in the auditory system. Biol Cybern. 2003;89:363–370. doi: 10.1007/s00422-003-0441-7. [DOI] [PubMed] [Google Scholar]
- Kraushaar U, Backus KH. Characterization of GABA(A) and glycine receptors in neurons of the developing rat inferior colliculus. Pflugers Arch. 2002;445:279–288. doi: 10.1007/s00424-002-0924-8. [DOI] [PubMed] [Google Scholar]
- Lalwani AK, Han JJ, Castelein CM, Carvalho GJ, Mhatre AN. In vitro and in vivo assessment of the ability of adeno-associated virus-brain-derived neurotrophic factor to enhance spiral ganglion cell survival following ototoxic insult. Laryngoscope. 2002;112:1325–1334. doi: 10.1097/00005537-200208000-00001. [DOI] [PubMed] [Google Scholar]
- Legendre P, Muller E, Badiu CI, Meier J, Vannier C, Triller A. Desensitization of homomeric alpha1 glycine receptor increases with receptor density. Mol Pharmacol. 2002;62:817–827. doi: 10.1124/mol.62.4.817. [DOI] [PubMed] [Google Scholar]
- Lessmann V. Neurotrophin-dependent modulation of glutamatergic synaptic transmission in the mammalian CNS. Gen Pharmacol. 1998;31:667–674. doi: 10.1016/s0306-3623(98)00190-6. [DOI] [PubMed] [Google Scholar]
- Lin X, Chen S, Chen P. Activation of metabotropic GABAB receptors inhibited glutamate responses in spiral ganglion neurons of mice. Neuroreport. 2000;11:957–961. doi: 10.1097/00001756-200004070-00012. [DOI] [PubMed] [Google Scholar]
- Liu Y, Ford B, Mann MA, Fischbach GD. Neuregulins increase alpha7 nicotinic acetylcholine receptors and enhance excitatory synaptic transmission in GABAergic interneurons of the hippocampus. J Neurosci. 2001;21:5660–5669. doi: 10.1523/JNEUROSCI.21-15-05660.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu VB, Moran TD, Balasubramanyan S, Alier KA, Dryden WF, Colmers WF, Smith PA. Substantia Gelatinosa neurons in defined-medium organotypic slice culture are similar to those in acute slices from young adult rats. Pain. 2006;121:261–275. doi: 10.1016/j.pain.2006.01.009. [DOI] [PubMed] [Google Scholar]
- McFadden SL, Ding D, Jiang H, Woo JM, Salvi RJ. Chinchilla models of selective cochlear hair cell loss. Hear Res. 2002;174:230–238. doi: 10.1016/s0378-5955(02)00697-4. [DOI] [PubMed] [Google Scholar]
- McGuinness SL, Shepherd RK. Exogenous BDNF rescues rat spiral ganglion neurons in vivo. Otol Neurotol. 2005;26:1064–1072. doi: 10.1097/01.mao.0000185063.20081.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchan-Perez A, Gil-Loyzaga P, Eybalin M. Ontogeny of glutamate decarboxylase and gamma-aminobutyric acid immunoreactivities in the rat cochlea. Eur Arch Otorhinolaryngol. 1990;248:4–7. doi: 10.1007/BF00634770. [DOI] [PubMed] [Google Scholar]
- Miller JM, Chi DH, O’Keeffe LJ, Kruszka P, Raphael Y, Altschuler RA. Neurotrophins can enhance spiral ganglion cell survival after inner hair cell loss. Int J Dev Neurosci. 1997;15:631–643. doi: 10.1016/s0736-5748(96)00117-7. [DOI] [PubMed] [Google Scholar]
- Mo ZL, Adamson CL, Davis RL. Dendrotoxin-sensitive K(+) currents contribute to accommodation in murine spiral ganglion neurons. J Physiol. 2002;542:763–778. doi: 10.1113/jphysiol.2002.017202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou K, Hunsberger CL, Cleary JM, Davis RL. Synergistic effects of BDNF and NT-3 on postnatal spiral ganglion neurons. J Comp Neurol. 1997;386:529–539. [PubMed] [Google Scholar]
- Nabekura J, Katsurabayashi S, Kakazu Y, Shibata S, Matsubara A, Jinno S, Mizoguchi Y, Sasaki A, Ishibashi H. Developmental switch from GABA to glycine release in single central synaptic terminals. Nat Neurosci. 2004;7:17–23. doi: 10.1038/nn1170. [DOI] [PubMed] [Google Scholar]
- Nadol JB., Jr Patterns of neural degeneration in the human cochlea and auditory nerve: implications for cochlear implantation. Otolaryngol Head Neck Surg. 1997;117:220–228. doi: 10.1016/s0194-5998(97)70178-5. [DOI] [PubMed] [Google Scholar]
- Narisawa-Saito M, Carnahan J, Araki K, Yamaguchi T, Nawa H. Brain-derived neurotrophic factor regulates the expression of AMPA receptor proteins in neocortical neurons. Neuroscience. 1999;88:1009–1014. doi: 10.1016/s0306-4522(98)00496-5. [DOI] [PubMed] [Google Scholar]
- Oestreicher E, Knipper M, Arnold A, Zenner HP, Felix D. Neurotrophin 3 potentiates glutamatergic responses of IHC afferents in the cochlea in vivo. Eur J Neurosci. 2000;12:1584–1590. doi: 10.1046/j.1460-9568.2000.00049.x. [DOI] [PubMed] [Google Scholar]
- Peng BG, Chen S, Lin X. Aspirin selectively augmented N-methyl-D-aspartate types of glutamate responses in cultured spiral ganglion neurons of mice. Neurosci Lett. 2003;343:21–24. doi: 10.1016/s0304-3940(03)00296-9. [DOI] [PubMed] [Google Scholar]
- Pisani A, Calabresi P, Centonze D, Bernardi G. Enhancement of NMDA responses by group I metabotropic glutamate receptor activation in striatal neurones. Br J Pharmacol. 1997;120:1007–1014. doi: 10.1038/sj.bjp.0700999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico B, Xu B, Reichardt LF. TrkB receptor signaling is required for establishment of GABAergic synapses in the cerebellum. Nat Neurosci. 2002;5:225–233. doi: 10.1038/nn808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieff HI, Raetzman LT, Sapp DW, Yeh HH, Siegel RE, Corfas G. Neuregulin induces GABA(A) receptor subunit expression and neurite outgrowth in cerebellar granule cells. J Neurosci. 1999;19:10757–10766. doi: 10.1523/JNEUROSCI.19-24-10757.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruel J, Chen C, Pujol R, Bobbin RP, Puel JL. AMPA-preferring glutamate receptors in cochlear physiology of adult guinea-pig. J Physiol (Lond) 1999;518:667–680. doi: 10.1111/j.1469-7793.1999.0667p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage C, Huang M, Karimi K, Gutierrez G, Vollrath MA, Zhang DS, Garcia-Anoveros J, Hinds PW, Corwin JT, Corey DP, Chen ZY. Proliferation of functional hair cells in vivo in the absence of the retinoblastoma protein. Science. 2005;307:1114–1118. doi: 10.1126/science.1106642. [DOI] [PubMed] [Google Scholar]
- Sah DW, Ray J, Gage FH. Regulation of voltage-and ligand-gated currents in rat hippocampal progenitor cells in vitro. J Neurobiol. 1997;32:95–110. doi: 10.1002/(sici)1097-4695(199701)32:1<95::aid-neu9>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Sergeeva OA, Haas HL. Expression and function of glycine receptors in striatal cholinergic interneurons from rat and mouse. Neuroscience. 2001;104:1043–1055. doi: 10.1016/s0306-4522(01)00130-0. [DOI] [PubMed] [Google Scholar]
- Staecker H, Galinovic-Schwartz V, Liu W, Lefebvre P, Kopke R, Malgrange B, Moonen G, Van De Water TR. The role of the neurotrophins in maturation and maintenance of postnatal auditory innervation. American Journal of Otology. 1996a;17:486–492. [PubMed] [Google Scholar]
- Staecker H, Kopke R, Malgrange B, Lefebvre P, Van de Water TR. NT-3 and/or BDNF therapy prevents loss of auditory neurons following loss of hair cells. Neuroreport. 1996b;7:889–894. doi: 10.1097/00001756-199603220-00011. [DOI] [PubMed] [Google Scholar]
- Stankovic KM, Corfas G. Real-time quantitative RT-PCR for low-abundance transcripts in the inner ear: analysis of neurotrophic factor expression. Hear Res. 2003;185:97–108. doi: 10.1016/s0378-5955(03)00298-3. [DOI] [PubMed] [Google Scholar]
- Sugawara M, Murtie JC, Stankovic KM, Liberman MC, Corfas G. Dynamic patterns of neurotrophin 3 expression in the postnatal mouse inner ear. J Comp Neurol. 2007;501:30–37. doi: 10.1002/cne.21227. [DOI] [PubMed] [Google Scholar]
- Tao L, Ye JH. Protein kinase C modulation of ethanol inhibition of glycine-activated current in dissociated neurons of rat ventral tegmental area. J Pharmacol Exp Ther. 2002;300:967–975. doi: 10.1124/jpet.300.3.967. [DOI] [PubMed] [Google Scholar]
- Tapia JC, Cardenas AM, Nualart F, Mentis GZ, Navarrete R, Aguayo LG. Neurite outgrowth in developing mouse spinal cord neurons is modulated by glycine receptors. Neuroreport. 2000;11:3007–3010. doi: 10.1097/00001756-200009110-00036. [DOI] [PubMed] [Google Scholar]
- Xiong H, Futamura T, Jourdi H, Zhou H, Takei N, Diverse-Pierluissi M, Plevy S, Nawa H. Neurotrophins induce BDNF expression through the glutamate receptor pathway in neocortical neurons. Neuropharmacology. 2002;42:903–912. doi: 10.1016/s0028-3908(02)00043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada MK, Nakanishi K, Ohba S, Nakamura T, Ikegaya Y, Nishiyama N, Matsuki N. Brain-derived neurotrophic factor promotes the maturation of GABAergic mechanisms in cultured hippocampal neurons. J Neurosci. 2002;22:7580–7585. doi: 10.1523/JNEUROSCI.22-17-07580.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Pollak GD. GABA and glycine have different effects on monaural response properties in the dorsal nucleus of the lateral lemniscus of the mustache bat. J Neurophysiol. 1994;71:2014–2024. doi: 10.1152/jn.1994.71.6.2014. [DOI] [PubMed] [Google Scholar]
- Zheng JL, Stewart RR, Gao WQ. Neurotrophin-4/5, brain-derived neurotrophic factor, and neurotrophin-3 promote survival of cultured vestibular ganglion neurons and protect them against neurotoxicity of ototoxins. J Neurobiol. 1995;28:330–340. doi: 10.1002/neu.480280306. [DOI] [PubMed] [Google Scholar]