Figure 2.
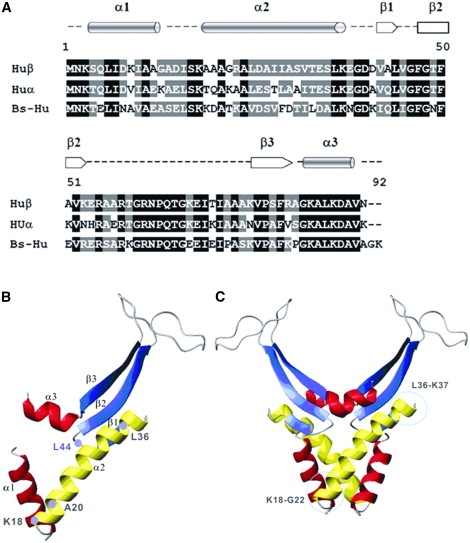
Structures of E. coli HUβ, E. coli HUα, and B. subtilis HU. (A) Alignment of the sequences using Vector NTI Advance 9. Residues identical in all sequences are highlighted in black. Similar residues are highlighted in gray. The secondary structure of the sequence is shown above the primary sequence. (B) The tertiary structure of the Ec-HUβ monomer composed of three α-helices and three β-sheets. Residues K18, A20, L36, and L44, which are part of the preferred cleavage sites, are indicated. The α1-helix and the α3-helix are shown in red, and the α2-helix is shown in yellow. The β-sheets are shown in blue. (C) The quaternary structure of the HUβ homodimer, which can be considered as the association of an α-subdomain and a β-subdomain (42). The DNA-binding domain is located in the β-subdomain. Peptide 9 is located in the α-subdomain.
