Figure 3.
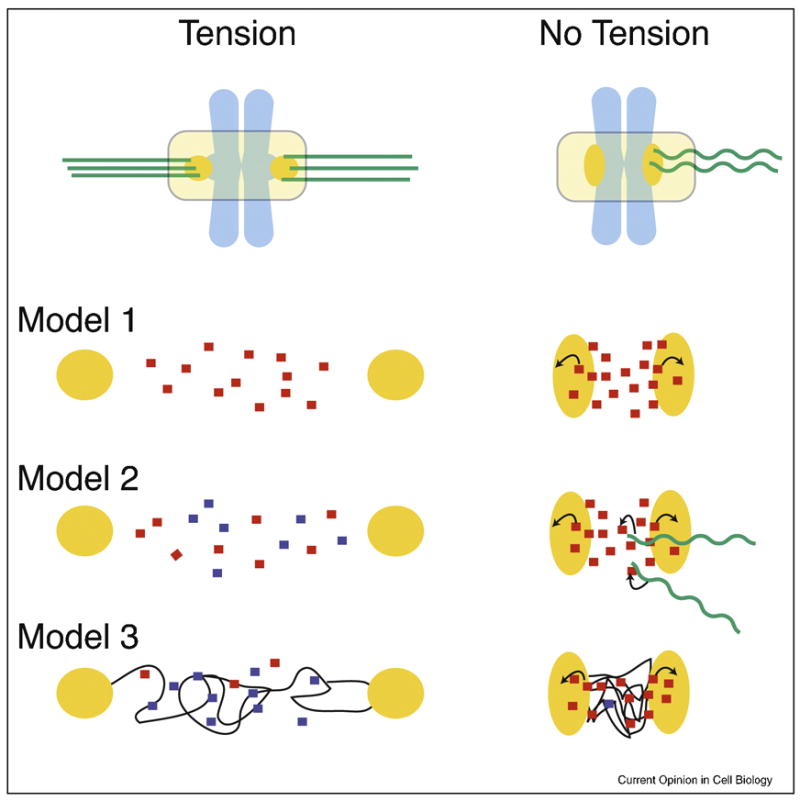
Three models of how the Aurora B pathway is controlled by microtubule attachment or tension.
Each model is a simplified enlargement of the inter-kinetochore regions of chromosomes under tension or in a relaxed state. Kinetochores are in orange, microtubules in green, and chromosomes are in light blue. Arrows indicate activation.
Model 1: Tension-regulated separation of Aurora B from its kinetochore substrates
The distance between active Aurora B at inner centromeres and its kinetochore substrates increases upon bioriented attachment. This increased distance would decrease the likelihood of Aurora B binding kinetochore substrates, which is reinforced by PP1 phosphatase.
Model 2: Microtubule-dependent regulation of Aurora B
Inner centromeric Aurora B on misaligned chromosomes is more accessible to microtubules than at the metaphase plate. Interactions with microtubules stimulate the kinase activity of Aurora B resulting destabilized microtubule attachments.
Model 3: Modulation of Aurora B activity through structural changes in centromeric chromatin
Assuming that the CPC is activated by chromatin through a clustering mechanism, the lack of tension may cause a compaction of chromatin at the centromere leading to kinase activation. However, under tension, the chromatin fibers are stretched resulting in a lower effective concentration of the CPC and thus activity. Chromatin fibers are modeled as thin black lines.
