Fig. 3.
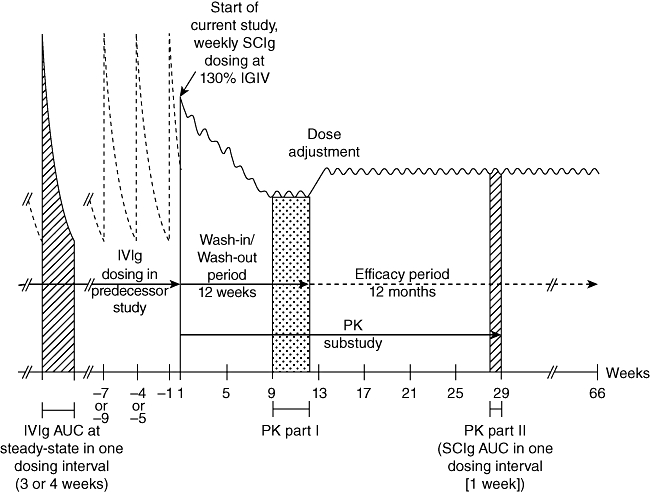
Study design of the US IgPro20 phase III study. The study consisted of a 12-week wash-in/wash-out period and a 12-month efficacy period.

Study design of the US IgPro20 phase III study. The study consisted of a 12-week wash-in/wash-out period and a 12-month efficacy period.