Abstract
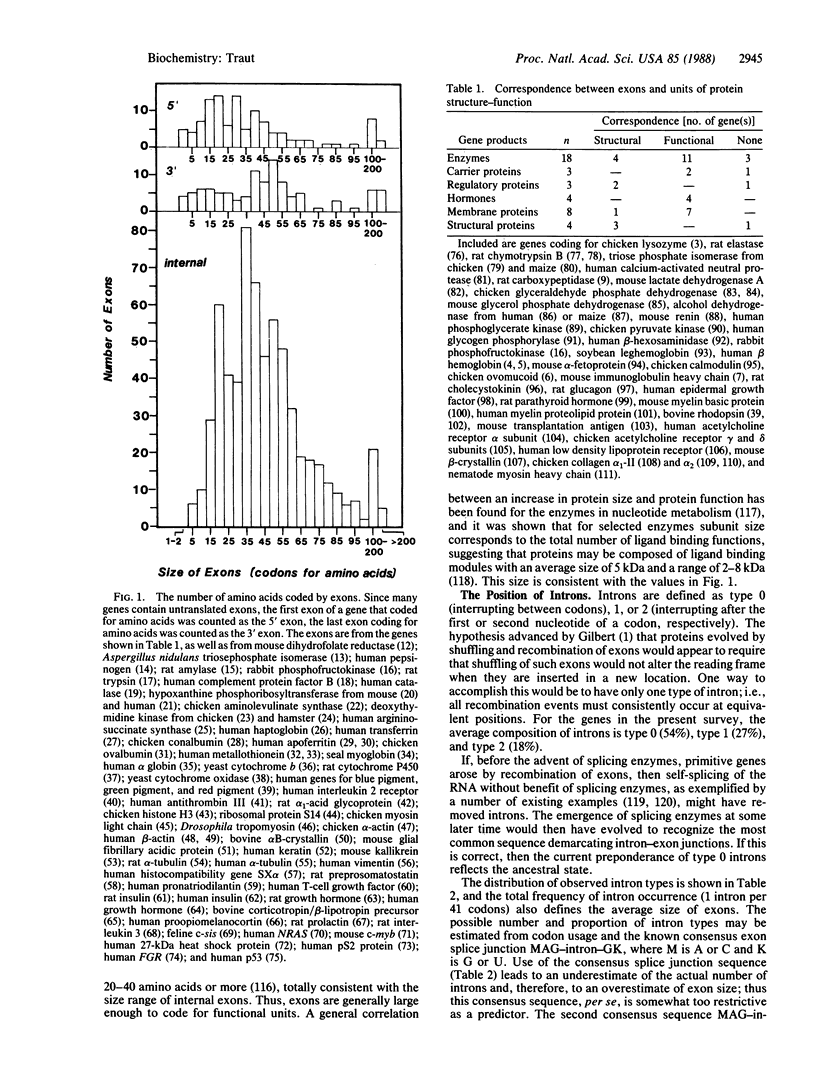
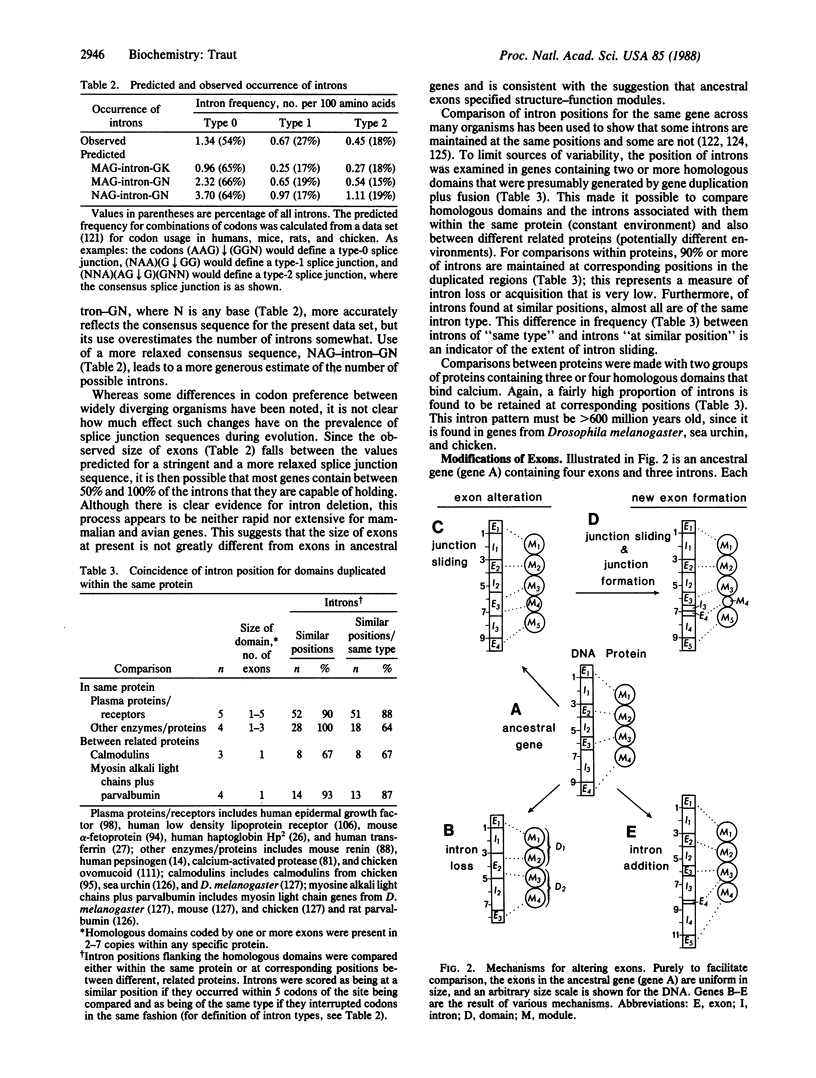
In considering the origin and evolution of proteins, the possibility that proteins evolved from exons coding for specific structure-function modules is attractive for its economy and simplicity but is not systematically supported by the available data. However, the number of correspondences between exons and units of protein structure-function that have so far been identified appears to be greater than expected by chance alone. The available data also show (i) that exons are fairly limited in size but are large enough to specify structure-function modules in proteins; (ii) that the position of introns for homologous domains in the same gene is reasonably stable, but there is also evidence for mechanisms that alter the position or existence of introns; and (iii) that it is possible that the observed relationship of exons to protein structure represents a degenerate state of an ancestral correspondence between exons and structure-function modules in proteins.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balcarek J. M., Cowan N. J. Structure of the mouse glial fibrillary acidic protein gene: implications for the evolution of the intermediate filament multigene family. Nucleic Acids Res. 1985 Aug 12;13(15):5527–5543. doi: 10.1093/nar/13.15.5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barta A., Richards R. I., Baxter J. D., Shine J. Primary structure and evolution of rat growth hormone gene. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4867–4871. doi: 10.1073/pnas.78.8.4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. I., Fong N. M., Stempien M. M., Wormsted M. A., Caput D., Ku L. L., Urdea M. S., Rall L. B., Sanchez-Pescador R. Human epidermal growth factor precursor: cDNA sequence, expression in vitro and gene organization. Nucleic Acids Res. 1986 Nov 11;14(21):8427–8446. doi: 10.1093/nar/14.21.8427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. I., Quinto C., Quiroga M., Valenzuela P., Craik C. S., Rutter W. J. Isolation and sequence of a rat chymotrypsin B gene. J Biol Chem. 1984 Nov 25;259(22):14265–14270. [PubMed] [Google Scholar]
- Berchtold M. W., Epstein P., Beaudet A. L., Payne M. E., Heizmann C. W., Means A. R. Structural organization and chromosomal assignment of the parvalbumin gene. J Biol Chem. 1987 Jun 25;262(18):8696–8701. [PubMed] [Google Scholar]
- Blanchetot A., Wilson V., Wood D., Jeffreys A. J. The seal myoglobin gene: an unusually long globin gene. Nature. 1983 Feb 24;301(5902):732–734. doi: 10.1038/301732a0. [DOI] [PubMed] [Google Scholar]
- Bonitz S. G., Coruzzi G., Thalenfeld B. E., Tzagoloff A., Macino G. Assembly of the mitochondrial membrane system. Structure and nucleotide sequence of the gene coding for subunit 1 of yeast cytochrme oxidase. J Biol Chem. 1980 Dec 25;255(24):11927–11941. [PubMed] [Google Scholar]
- Boss J. M., Mengler R., Okada K., Auffray C., Strominger J. L. Sequence analysis of the human major histocompatibility gene SX alpha. Mol Cell Biol. 1985 Oct;5(10):2677–2683. doi: 10.1128/mcb.5.10.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brush D., Dodgson J. B., Choi O. R., Stevens P. W., Engel J. D. Replacement variant histone genes contain intervening sequences. Mol Cell Biol. 1985 Jun;5(6):1307–1317. doi: 10.1128/mcb.5.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brändén C. I., Eklund H., Cambillau C., Pryor A. J. Correlation of exons with structural domains in alcohol dehydrogenase. EMBO J. 1984 Jun;3(6):1307–1310. doi: 10.1002/j.1460-2075.1984.tb01967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke J., Hwang P., Anderson L., Lebo R., Gorin F., Fletterick R. Intron/exon structure of the human gene for the muscle isozyme of glycogen phosphorylase. Proteins. 1987;2(3):177–187. doi: 10.1002/prot.340020303. [DOI] [PubMed] [Google Scholar]
- Campbell R. D., Porter R. R. Molecular cloning and characterization of the gene coding for human complement protein factor B. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4464–4468. doi: 10.1073/pnas.80.14.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien Y. H., Thompson E. B. Genomic organization of rat prolactin and growth hormone genes. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4583–4587. doi: 10.1073/pnas.77.8.4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochet M., Chang A. C., Cohen S. N. Characterization of the structural gene and putative 5'-regulatory sequences for human proopiomelanocortin. Nature. 1982 May 27;297(5864):335–339. doi: 10.1038/297335a0. [DOI] [PubMed] [Google Scholar]
- Cochet M., Gannon F., Hen R., Maroteaux L., Perrin F., Chambon P. Organization and sequence studies of the 17-piece chicken conalbumin gene. Nature. 1979 Dec 6;282(5739):567–574. doi: 10.1038/282567a0. [DOI] [PubMed] [Google Scholar]
- Cohen D. R., Hapel A. J., Young I. G. Cloning and expression of the rat interleukin-3 gene. Nucleic Acids Res. 1986 May 12;14(9):3641–3658. doi: 10.1093/nar/14.9.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo F., Colombo M., Staempfli S., Santoro C., Marone M., Frank R., Delius H., Cortese R. Structure of gene and pseudogenes of human apoferritin H. Nucleic Acids Res. 1986 Jan 24;14(2):721–736. doi: 10.1093/nar/14.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik C. S., Buchman S. R., Beychok S. O2 binding properties of the product of the central exon of beta-globin gene. Nature. 1981 May 7;291(5810):87–90. doi: 10.1038/291087a0. [DOI] [PubMed] [Google Scholar]
- Craik C. S., Choo Q. L., Swift G. H., Quinto C., MacDonald R. J., Rutter W. J. Structure of two related rat pancreatic trypsin genes. J Biol Chem. 1984 Nov 25;259(22):14255–14264. [PubMed] [Google Scholar]
- Craik C. S., Rutter W. J., Fletterick R. Splice junctions: association with variation in protein structure. Science. 1983 Jun 10;220(4602):1125–1129. doi: 10.1126/science.6344214. [DOI] [PubMed] [Google Scholar]
- Craik C. S., Sprang S., Fletterick R., Rutter W. J. Intron-exon splice junctions map at protein surfaces. Nature. 1982 Sep 9;299(5879):180–182. doi: 10.1038/299180a0. [DOI] [PubMed] [Google Scholar]
- DeNoto F. M., Moore D. D., Goodman H. M. Human growth hormone DNA sequence and mRNA structure: possible alternative splicing. Nucleic Acids Res. 1981 Aug 11;9(15):3719–3730. doi: 10.1093/nar/9.15.3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes R. J., Haun R. S., Funckes C. L., Dixon J. E. A gene encoding rat cholecystokinin. Isolation, nucleotide sequence, and promoter activity. J Biol Chem. 1985 Jan 25;260(2):1280–1286. [PubMed] [Google Scholar]
- Diehl H. J., Schaich M., Budzinski R. M., Stoffel W. Individual exons encode the integral membrane domains of human myelin proteolipid protein. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9807–9811. doi: 10.1073/pnas.83.24.9807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duester G., Jörnvall H., Hatfield G. W. Intron-dependent evolution of the nucleotide-binding domains within alcohol dehydrogenase and related enzymes. Nucleic Acids Res. 1986 Mar 11;14(5):1931–1941. doi: 10.1093/nar/14.5.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton W. A. The relationship between coding sequences and function in haemoglobin. Nature. 1980 Mar 13;284(5752):183–185. doi: 10.1038/284183a0. [DOI] [PubMed] [Google Scholar]
- Eiferman F. A., Young P. R., Scott R. W., Tilghman S. M. Intragenic amplification and divergence in the mouse alpha-fetoprotein gene. Nature. 1981 Dec 24;294(5843):713–718. doi: 10.1038/294713a0. [DOI] [PubMed] [Google Scholar]
- Ferrari S., Battini R., Kaczmarek L., Rittling S., Calabretta B., de Riel J. K., Philiponis V., Wei J. F., Baserga R. Coding sequence and growth regulation of the human vimentin gene. Mol Cell Biol. 1986 Nov;6(11):3614–3620. doi: 10.1128/mcb.6.11.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornwald J. A., Kuncio G., Peng I., Ordahl C. P. The complete nucleotide sequence of the chick a-actin gene and its evolutionary relationship to the actin gene family. Nucleic Acids Res. 1982 Jul 10;10(13):3861–3876. doi: 10.1093/nar/10.13.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freytag S. O., Beaudet A. L., Bock H. G., O'Brien W. E. Molecular structure of the human argininosuccinate synthetase gene: occurrence of alternative mRNA splicing. Mol Cell Biol. 1984 Oct;4(10):1978–1984. doi: 10.1128/mcb.4.10.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W., Marchionni M., McKnight G. On the antiquity of introns. Cell. 1986 Jul 18;46(2):151–153. doi: 10.1016/0092-8674(86)90730-0. [DOI] [PubMed] [Google Scholar]
- Gilbert W. Why genes in pieces? Nature. 1978 Feb 9;271(5645):501–501. doi: 10.1038/271501a0. [DOI] [PubMed] [Google Scholar]
- Greenberg B. D., Bencen G. H., Seilhamer J. J., Lewicki J. A., Fiddes J. C. Nucleotide sequence of the gene encoding human atrial natriuretic factor precursor. Nature. 1984 Dec 13;312(5995):656–658. doi: 10.1038/312656a0. [DOI] [PubMed] [Google Scholar]
- Hall A., Brown R. Human N-ras: cDNA cloning and gene structure. Nucleic Acids Res. 1985 Jul 25;13(14):5255–5268. doi: 10.1093/nar/13.14.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J. L., Cowan N. J. Structural features and restricted expression of a human alpha-tubulin gene. Nucleic Acids Res. 1985 Jan 11;13(1):207–223. doi: 10.1093/nar/13.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heguy A., West A., Richards R. I., Karin M. Structure and tissue-specific expression of the human metallothionein IB gene. Mol Cell Biol. 1986 Jun;6(6):2149–2157. doi: 10.1128/mcb.6.6.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig R., Perrin F., Gannon F., Mandel J. L., Chambon P. The ovalbumin gene family: structure of the X gene and evolution of duplicated split genes. Cell. 1980 Jul;20(3):625–637. doi: 10.1016/0092-8674(80)90309-8. [DOI] [PubMed] [Google Scholar]
- Heinrich G., Gros P., Habener J. F. Glucagon gene sequence. Four of six exons encode separate functional domains of rat pre-proglucagon. J Biol Chem. 1984 Nov 25;259(22):14082–14087. [PubMed] [Google Scholar]
- Heinrich G., Kronenberg H. M., Potts J. T., Jr, Habener J. F. Gene encoding parathyroid hormone. Nucleotide sequence of the rat gene and deduced amino acid sequence of rat preproparathyroid hormone. J Biol Chem. 1984 Mar 10;259(5):3320–3329. [PubMed] [Google Scholar]
- Hickey E., Brandon S. E., Potter R., Stein G., Stein J., Weber L. A. Sequence and organization of genes encoding the human 27 kDa heat shock protein. Nucleic Acids Res. 1986 May 27;14(10):4127–4145. doi: 10.1093/nar/14.10.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook N. J., Smith K. A., Fornace A. J., Jr, Comeau C. M., Wiskocil R. L., Crabtree G. R. T-cell growth factor: complete nucleotide sequence and organization of the gene in normal and malignant cells. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1634–1638. doi: 10.1073/pnas.81.6.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm I., Ollo R., Panthier J. J., Rougeon F. Evolution of aspartyl proteases by gene duplication: the mouse renin gene is organized in two homologous clusters of four exons. EMBO J. 1984 Mar;3(3):557–562. doi: 10.1002/j.1460-2075.1984.tb01846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M. C., Sharp S. B., Davidson N. The complete sequence of the mouse skeletal alpha-actin gene reveals several conserved and inverted repeat sequences outside of the protein-coding region. Mol Cell Biol. 1986 Jan;6(1):15–25. doi: 10.1128/mcb.6.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inana G., Piatigorsky J., Norman B., Slingsby C., Blundell T. Gene and protein structure of a beta-crystallin polypeptide in murine lens: relationship of exons and structural motifs. Nature. 1983 Mar 24;302(5906):310–315. doi: 10.1038/302310a0. [DOI] [PubMed] [Google Scholar]
- Ireland R. C., Kotarski M. A., Johnston L. A., Stadler U., Birkenmeier E., Kozak L. P. Primary structure of the mouse glycerol-3-phosphate dehydrogenase gene. J Biol Chem. 1986 Sep 5;261(25):11779–11785. [PubMed] [Google Scholar]
- Ishida N., Kanamori H., Noma T., Nikaido T., Sabe H., Suzuki N., Shimizu A., Honjo T. Molecular cloning and structure of the human interleukin 2 receptor gene. Nucleic Acids Res. 1985 Nov 11;13(21):7579–7589. doi: 10.1093/nar/13.21.7579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janin J., Wodak S. J. Structural domains in proteins and their role in the dynamics of protein function. Prog Biophys Mol Biol. 1983;42(1):21–78. doi: 10.1016/0079-6107(83)90003-2. [DOI] [PubMed] [Google Scholar]
- Jeltsch J. M., Roberts M., Schatz C., Garnier J. M., Brown A. M., Chambon P. Structure of the human oestrogen-responsive gene pS2. Nucleic Acids Res. 1987 Feb 25;15(4):1401–1414. doi: 10.1093/nar/15.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. D., Idler W. W., Zhou X. M., Roop D. R., Steinert P. M. Structure of a gene for the human epidermal 67-kDa keratin. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1896–1900. doi: 10.1073/pnas.82.7.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung A., Sippel A. E., Grez M., Schütz G. Exons encode functional and structural units of chicken lysozyme. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5759–5763. doi: 10.1073/pnas.77.10.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaine B. P., Gupta R., Woese C. R. Putative introns in tRNA genes of prokaryotes. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3309–3312. doi: 10.1073/pnas.80.11.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M., Richards R. I. Human metallothionein genes--primary structure of the metallothionein-II gene and a related processed gene. Nature. 1982 Oct 28;299(5886):797–802. doi: 10.1038/299797a0. [DOI] [PubMed] [Google Scholar]
- Karlik C. C., Fyrberg E. A. Two Drosophila melanogaster tropomyosin genes: structural and functional aspects. Mol Cell Biol. 1986 Jun;6(6):1965–1973. doi: 10.1128/mcb.6.6.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karn J., Dibb N. J., Miller D. M. Cloning nematode myosin genes. Cell Muscle Motil. 1985;6:185–237. doi: 10.1007/978-1-4757-4723-2_7. [DOI] [PubMed] [Google Scholar]
- Kim S. H., Moores J. C., David D., Respess J. G., Jolly D. J., Friedmann T. The organization of the human HPRT gene. Nucleic Acids Res. 1986 Apr 11;14(7):3103–3118. doi: 10.1093/nar/14.7.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb P., Crawford L. Characterization of the human p53 gene. Mol Cell Biol. 1986 May;6(5):1379–1385. doi: 10.1128/mcb.6.5.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavu S., Reddy E. P. Structural organization and nucleotide sequence of mouse c-myb oncogene: activation in ABPL tumors is due to viral integration in an intron which results in the deletion of the 5' coding sequences. Nucleic Acids Res. 1986 Jul 11;14(13):5309–5320. doi: 10.1093/nar/14.13.5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. P., Kao M. C., French B. A., Putney S. D., Chang S. H. The rabbit muscle phosphofructokinase gene. Implications for protein structure, function, and tissue specificity. J Biol Chem. 1987 Mar 25;262(9):4195–4199. [PubMed] [Google Scholar]
- Leicht M., Long G. L., Chandra T., Kurachi K., Kidd V. J., Mace M., Jr, Davie E. W., Woo S. L. Sequence homology and structural comparison between the chromosomal human alpha 1-antitrypsin and chicken ovalbumin genes. Nature. 1982 Jun 24;297(5868):655–659. doi: 10.1038/297655a0. [DOI] [PubMed] [Google Scholar]
- Lemischka I., Sharp P. A. The sequences of an expressed rat alpha-tubulin gene and a pseudogene with an inserted repetitive element. Nature. 1982 Nov 25;300(5890):330–335. doi: 10.1038/300330a0. [DOI] [PubMed] [Google Scholar]
- Lewin R. Surprise finding with insect globin genes. Science. 1984 Oct 19;226(4672):328–328. doi: 10.1126/science.226.4672.328. [DOI] [PubMed] [Google Scholar]
- Lewis J. A. Structure and expression of the Chinese hamster thymidine kinase gene. Mol Cell Biol. 1986 Jun;6(6):1998–2010. doi: 10.1128/mcb.6.6.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. S., Tiano H. F., Fukasawa K. M., Yagi K., Shimizu M., Sharief F. S., Nakashima Y., Pan Y. E. Protein structure and gene organization of mouse lactate dehydrogenase-A isozyme. Eur J Biochem. 1985 Jun 3;149(2):215–225. doi: 10.1111/j.1432-1033.1985.tb08914.x. [DOI] [PubMed] [Google Scholar]
- Liao Y. C., Taylor J. M., Vannice J. L., Clawson G. A., Smuckler E. A. Structure of the rat alpha 1-acid glycoprotein gene. Mol Cell Biol. 1985 Dec;5(12):3634–3639. doi: 10.1128/mcb.5.12.3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomedico P., Rosenthal N., Efstratidadis A., Gilbert W., Kolodner R., Tizard R. The structure and evolution of the two nonallelic rat preproinsulin genes. Cell. 1979 Oct;18(2):545–558. doi: 10.1016/0092-8674(79)90071-0. [DOI] [PubMed] [Google Scholar]
- Lonberg N., Gilbert W. Intron/exon structure of the chicken pyruvate kinase gene. Cell. 1985 Jan;40(1):81–90. doi: 10.1016/0092-8674(85)90311-3. [DOI] [PubMed] [Google Scholar]
- MacDonald R. J., Crerar M. M., Swain W. F., Pictet R. L., Thomas G., Rutter W. J. Structure of a family of rat amylase genes. Nature. 1980 Sep 11;287(5778):117–122. doi: 10.1038/287117a0. [DOI] [PubMed] [Google Scholar]
- Maeda N., Yang F., Barnett D. R., Bowman B. H., Smithies O. Duplication within the haptoglobin Hp2 gene. Nature. 1984 May 10;309(5964):131–135. doi: 10.1038/309131a0. [DOI] [PubMed] [Google Scholar]
- Maguire D. J., Day A. R., Borthwick I. A., Srivastava G., Wigley P. L., May B. K., Elliott W. H. Nucleotide sequence of the chicken 5-aminolevulinate synthase gene. Nucleic Acids Res. 1986 Feb 11;14(3):1379–1391. doi: 10.1093/nar/14.3.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchionni M., Gilbert W. The triosephosphate isomerase gene from maize: introns antedate the plant-animal divergence. Cell. 1986 Jul 4;46(1):133–141. doi: 10.1016/0092-8674(86)90867-6. [DOI] [PubMed] [Google Scholar]
- Mason A. J., Evans B. A., Cox D. R., Shine J., Richards R. I. Structure of mouse kallikrein gene family suggests a role in specific processing of biologically active peptides. Nature. 1983 May 26;303(5915):300–307. doi: 10.1038/303300a0. [DOI] [PubMed] [Google Scholar]
- McKnight G. L., O'Hara P. J., Parker M. L. Nucleotide sequence of the triosephosphate isomerase gene from Aspergillus nidulans: implications for a differential loss of introns. Cell. 1986 Jul 4;46(1):143–147. doi: 10.1016/0092-8674(86)90868-8. [DOI] [PubMed] [Google Scholar]
- Merrill G. F., Harland R. M., Groudine M., McKnight S. L. Genetic and physical analysis of the chicken tk gene. Mol Cell Biol. 1984 Sep;4(9):1769–1776. doi: 10.1128/mcb.4.9.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson A. M., Blake C. C., Evans S. T., Orkin S. H. Structure of the human phosphoglycerate kinase gene and the intron-mediated evolution and dispersal of the nucleotide-binding domain. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6965–6969. doi: 10.1073/pnas.82.20.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake S., Emori Y., Suzuki K. Gene organization of the small subunit of human calcium-activated neutral protease. Nucleic Acids Res. 1986 Nov 25;14(22):8805–8817. doi: 10.1093/nar/14.22.8805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizukami Y., Sogawa K., Suwa Y., Muramatsu M., Fujii-Kuriyama Y. Gene structure of a phenobarbital-inducible cytochrome P-450 in rat liver. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3958–3962. doi: 10.1073/pnas.80.13.3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montminy M. R., Goodman R. H., Horovitch S. J., Habener J. F. Primary structure of the gene encoding rat preprosomatostatin. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3337–3340. doi: 10.1073/pnas.81.11.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabeshima Y., Fujii-Kuriyama Y., Muramatsu M., Ogata K. Alternative transcription and two modes of splicing results in two myosin light chains from one gene. Nature. 1984 Mar 22;308(5957):333–338. doi: 10.1038/308333a0. [DOI] [PubMed] [Google Scholar]
- Nakajima-Iijima S., Hamada H., Reddy P., Kakunaga T. Molecular structure of the human cytoplasmic beta-actin gene: interspecies homology of sequences in the introns. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6133–6137. doi: 10.1073/pnas.82.18.6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi S., Teranishi Y., Watanabe Y., Notake M., Noda M., Kakidani H., Jingami H., Numa S. Isolation and characterization of the bovine corticotropin/beta-lipotropin precursor gene. Eur J Biochem. 1981 Apr;115(3):429–438. doi: 10.1111/j.1432-1033.1981.tb06220.x. [DOI] [PubMed] [Google Scholar]
- Naora H., Deacon N. J. Relationship between the total size of exons and introns in protein-coding genes of higher eukaryotes. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6196–6200. doi: 10.1073/pnas.79.20.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathans J., Hogness D. S. Isolation, sequence analysis, and intron-exon arrangement of the gene encoding bovine rhodopsin. Cell. 1983 Oct;34(3):807–814. doi: 10.1016/0092-8674(83)90537-8. [DOI] [PubMed] [Google Scholar]
- Nathans J., Thomas D., Hogness D. S. Molecular genetics of human color vision: the genes encoding blue, green, and red pigments. Science. 1986 Apr 11;232(4747):193–202. doi: 10.1126/science.2937147. [DOI] [PubMed] [Google Scholar]
- Nef P., Mauron A., Stalder R., Alliod C., Ballivet M. Structure linkage, and sequence of the two genes encoding the delta and gamma subunits of the nicotinic acetylcholine receptor. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7975–7979. doi: 10.1073/pnas.81.24.7975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemer M., Chamberland M., Sirois D., Argentin S., Drouin J., Dixon R. A., Zivin R. A., Condra J. H. Gene structure of human cardiac hormone precursor, pronatriodilatin. Nature. 1984 Dec 13;312(5995):654–656. doi: 10.1038/312654a0. [DOI] [PubMed] [Google Scholar]
- Nishizawa M., Semba K., Yoshida M. C., Yamamoto T., Sasaki M., Toyoshima K. Structure, expression, and chromosomal location of the human c-fgr gene. Mol Cell Biol. 1986 Feb;6(2):511–517. doi: 10.1128/mcb.6.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobrega F. G., Tzagoloff A. Assembly of the mitochondrial membrane system. DNA sequence and organization of the cytochrome b gene in Saccharomyces cerevisiae D273-10B. J Biol Chem. 1980 Oct 25;255(20):9828–9837. [PubMed] [Google Scholar]
- Noda M., Furutani Y., Takahashi H., Toyosato M., Tanabe T., Shimizu S., Kikyotani S., Kayano T., Hirose T., Inayama S. Cloning and sequence analysis of calf cDNA and human genomic DNA encoding alpha-subunit precursor of muscle acetylcholine receptor. 1983 Oct 27-Nov 2Nature. 305(5937):818–823. doi: 10.1038/305818a0. [DOI] [PubMed] [Google Scholar]
- Nunberg J. H., Kaufman R. J., Chang A. C., Cohen S. N., Schimke R. T. Structure and genomic organization of the mouse dihydrofolate reductase gene. Cell. 1980 Feb;19(2):355–364. doi: 10.1016/0092-8674(80)90510-3. [DOI] [PubMed] [Google Scholar]
- Ohkubo H., Vogeli G., Mudryj M., Avvedimento V. E., Sullivan M., Pastan I., de Crombrugghe B. Isolation and characterization of overlapping genomic clones covering the chicken alpha 2 (type I) collagen gene. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7059–7063. doi: 10.1073/pnas.77.12.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park I., Schaeffer E., Sidoli A., Baralle F. E., Cohen G. N., Zakin M. M. Organization of the human transferrin gene: direct evidence that it originated by gene duplication. Proc Natl Acad Sci U S A. 1985 May;82(10):3149–3153. doi: 10.1073/pnas.82.10.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P. I., Framson P. E., Caskey C. T., Chinault A. C. Fine structure of the human hypoxanthine phosphoribosyltransferase gene. Mol Cell Biol. 1986 Feb;6(2):393–403. doi: 10.1128/mcb.6.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochownik E. V., Bock S. C., Orkin S. H. Intron structure of the human antithrombin III gene differs from that of other members of the serine protease inhibitor superfamily. J Biol Chem. 1985 Aug 15;260(17):9608–9612. [PubMed] [Google Scholar]
- Proia R. L., Soravia E. Organization of the gene encoding the human beta-hexosaminidase alpha-chain. J Biol Chem. 1987 Apr 25;262(12):5677–5681. [PubMed] [Google Scholar]
- Proudfoot N. J., Maniatis T. The structure of a human alpha-globin pseudogene and its relationship to alpha-globin gene duplication. Cell. 1980 Sep;21(2):537–544. doi: 10.1016/0092-8674(80)90491-2. [DOI] [PubMed] [Google Scholar]
- Quan F., Korneluk R. G., Tropak M. B., Gravel R. A. Isolation and characterization of the human catalase gene. Nucleic Acids Res. 1986 Jul 11;14(13):5321–5335. doi: 10.1093/nar/14.13.5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quax-Jeuken Y., Quax W., van Rens G., Khan P. M., Bloemendal H. Complete structure of the alpha B-crystallin gene: conservation of the exon-intron distribution in the two nonlinked alpha-crystallin genes. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5819–5823. doi: 10.1073/pnas.82.17.5819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinto C., Quiroga M., Swain W. F., Nikovits W. C., Jr, Standring D. N., Pictet R. L., Valenzuela P., Rutter W. J. Rat preprocarboxypeptidase A: cDNA sequence and preliminary characterization of the gene. Proc Natl Acad Sci U S A. 1982 Jan;79(1):31–35. doi: 10.1073/pnas.79.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads D. D., Dixit A., Roufa D. J. Primary structure of human ribosomal protein S14 and the gene that encodes it. Mol Cell Biol. 1986 Aug;6(8):2774–2783. doi: 10.1128/mcb.6.8.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakano H., Rogers J. H., Hüppi K., Brack C., Traunecker A., Maki R., Wall R., Tonegawa S. Domains and the hinge region of an immunoglobulin heavy chain are encoded in separate DNA segments. Nature. 1979 Feb 22;277(5698):627–633. doi: 10.1038/277627a0. [DOI] [PubMed] [Google Scholar]
- Santoro C., Marone M., Ferrone M., Costanzo F., Colombo M., Minganti C., Cortese R., Silengo L. Cloning of the gene coding for human L apoferritin. Nucleic Acids Res. 1986 Apr 11;14(7):2863–2876. doi: 10.1093/nar/14.7.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmen R. C., Tanaka T., Ts'ui K. F., Putkey J. A., Scott M. J., Lai E. C., Means A. R. The structural organization of the chicken calmodulin gene. J Biol Chem. 1985 Jan 25;260(2):907–912. [PubMed] [Google Scholar]
- Smith V. L., Doyle K. E., Maune J. F., Munjaal R. P., Beckingham K. Structure and sequence of the Drosophila melanogaster calmodulin gene. J Mol Biol. 1987 Aug 5;196(3):471–485. doi: 10.1016/0022-2836(87)90025-8. [DOI] [PubMed] [Google Scholar]
- Sogawa K., Fujii-Kuriyama Y., Mizukami Y., Ichihara Y., Takahashi K. Primary structure of human pepsinogen gene. J Biol Chem. 1983 Apr 25;258(8):5306–5311. [PubMed] [Google Scholar]
- Stein J. P., Catterall J. F., Kristo P., Means A. R., O'Malley B. W. Ovomucoid intervening sequences specify functional domains and generate protein polymorphism. Cell. 1980 Oct;21(3):681–687. doi: 10.1016/0092-8674(80)90431-6. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Moore K. W., Frelinger J. G., Sher B. T., Shen F. W., Boyse E. A., Hood L. A pseudogene homologous to mouse transplantation antigens: transplantation antigens are encoded by eight exons that correlate with protein domains. Cell. 1981 Sep;25(3):683–692. doi: 10.1016/0092-8674(81)90175-6. [DOI] [PubMed] [Google Scholar]
- Stone E. M., Rothblum K. N., Alevy M. C., Kuo T. M., Schwartz R. J. Complete sequence of the chicken glyceraldehyde-3-phosphate dehydrogenase gene. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1628–1632. doi: 10.1073/pnas.82.6.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone E. M., Rothblum K. N., Schwartz R. J. Intron-dependent evolution of chicken glyceraldehyde phosphate dehydrogenase gene. Nature. 1985 Feb 7;313(6002):498–500. doi: 10.1038/313498a0. [DOI] [PubMed] [Google Scholar]
- Straus D., Gilbert W. Genetic engineering in the Precambrian: structure of the chicken triosephosphate isomerase gene. Mol Cell Biol. 1985 Dec;5(12):3497–3506. doi: 10.1128/mcb.5.12.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift G. H., Craik C. S., Stary S. J., Quinto C., Lahaie R. G., Rutter W. J., MacDonald R. J. Structure of the two related elastase genes expressed in the rat pancreas. J Biol Chem. 1984 Nov 25;259(22):14271–14278. [PubMed] [Google Scholar]
- Südhof T. C., Goldstein J. L., Brown M. S., Russell D. W. The LDL receptor gene: a mosaic of exons shared with different proteins. Science. 1985 May 17;228(4701):815–822. doi: 10.1126/science.2988123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traut T. W. Are proteins made of modules? Mol Cell Biochem. 1986 Apr;70(1):3–10. doi: 10.1007/BF00233799. [DOI] [PubMed] [Google Scholar]
- Ueyama H., Hamada H., Battula N., Kakunaga T. Structure of a human smooth muscle actin gene (aortic type) with a unique intron site. Mol Cell Biol. 1984 Jun;4(6):1073–1078. doi: 10.1128/mcb.4.6.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upholt W. B., Sandell L. J. Exon/intron organization of the chicken type II procollagen gene: intron size distribution suggests a minimal intron size. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2325–2329. doi: 10.1073/pnas.83.8.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Ouweland A. M., Van Groningen J. J., Schalken J. A., Van Neck H. W., Bloemers H. P., Van de Ven W. J. Genetic organization of the c-sis transcription unit. Nucleic Acids Res. 1987 Feb 11;15(3):959–970. doi: 10.1093/nar/15.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetlaufer D. B. Folding of protein fragments. Adv Protein Chem. 1981;34:61–92. doi: 10.1016/s0065-3233(08)60518-5. [DOI] [PubMed] [Google Scholar]
- Wodak S. J., Janin J. Location of structural domains in protein. Biochemistry. 1981 Nov 10;20(23):6544–6552. doi: 10.1021/bi00526a005. [DOI] [PubMed] [Google Scholar]
- Zaug A. J., Cech T. R. The intervening sequence RNA of Tetrahymena is an enzyme. Science. 1986 Jan 31;231(4737):470–475. doi: 10.1126/science.3941911. [DOI] [PubMed] [Google Scholar]
- Zehfus M. H., Rose G. D. Compact units in proteins. Biochemistry. 1986 Sep 23;25(19):5759–5765. doi: 10.1021/bi00367a062. [DOI] [PubMed] [Google Scholar]
- de Ferra F., Engh H., Hudson L., Kamholz J., Puckett C., Molineaux S., Lazzarini R. A. Alternative splicing accounts for the four forms of myelin basic protein. Cell. 1985 Dec;43(3 Pt 2):721–727. doi: 10.1016/0092-8674(85)90245-4. [DOI] [PubMed] [Google Scholar]


