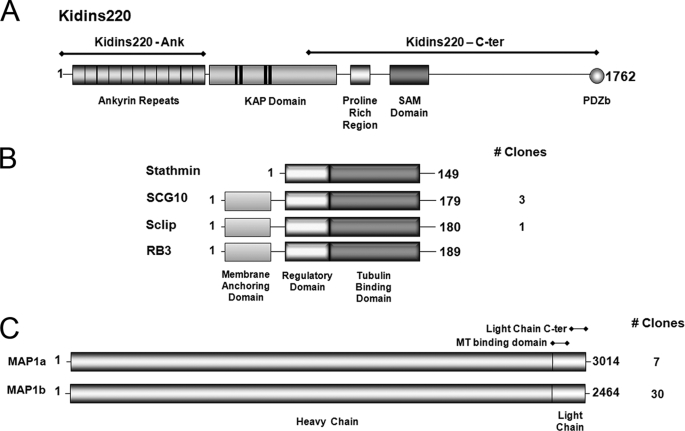
FIGURE 6.
Kidins220/ARMS domains used as baits in yeast two-hybrid assays and the interacting proteins identified. A, the 11 ankyrin repeats present at the Kidins220/ARMS N-terminal region (Kidins220-Ank) as well as its C-terminal region containing a proline-rich region, a SAM domain, and a PDZ binding motif (Kidins220-C-ter) were used as baits to screen a mouse brain cDNA library. B, schematic representation of the domain structure for the stathmin family members identified as Kidins220/ARMS-interacting proteins. All members contain the highly conserved regulatory and tubulin binding domains. SCG10, Sclip, and RB3 also possess an N-terminal membrane-anchoring domain absent in stathmin. Three independent clones of SCG10 and one clone of Sclip were identified. C, schematic representation of the domain structure for MAP1a and MAP1b. These proteins consist of a dimer between a heavy chain and a light chain derived from the same polypeptide. A very high number of clones of MAP1 were identified as Kinds220/ARMS-interacting proteins (37 in total).