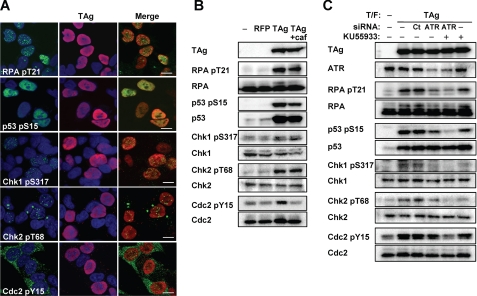
FIGURE 4.
Induction of ATM- and ATR-dependent G2 checkpoint signaling in TAg-expressing IMR-32 cells. A, IMR-32 cells were transfected with an expression vector for FLAG-TAg 3 days before double immunofluorescence staining with antibodies to the indicated phosphorylated forms of checkpoint proteins (green) and antibodies to TAg (red). Cell nuclei were stained with DAPI (blue). pS, phosphoserine; pY, phosphotyrosine; pT, phosphothreonine. Scale bars, 10 μm. B, IMR-32 cells transfected with expression vectors for FLAG-red fluorescent protein or FLAG-TAg, or with the corresponding empty vector (−), were incubated in the absence or presence of 2.5 mm caffeine (caf) for 15 h and then subjected to immunoblot analysis with antibodies to phosphorylated or total forms of the indicated proteins. C, IMR-32 cells transfected (T/F) with control (CT) or ATR siRNAs, as well as with an expression vector for FLAG-TAg or the corresponding empty vector (−), were incubated for 15 h in the absence or presence of the ATM inhibitor KU55933 (20 μm) and then subjected to immunoblot analysis with antibodies to phosphorylated or total forms of the indicated proteins.