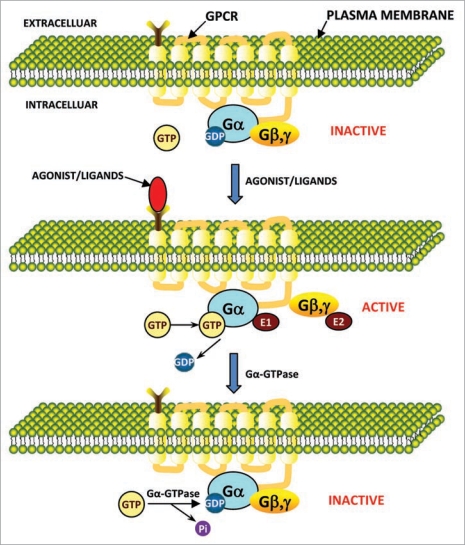
Figure 1.
Model for signal transduction by activation/inactivation of heterotrimeric G proteins through GPCR. The subunits of heterotrimeric G proteins (Gα and Gβγ) in their inactivated state are associated with each other. In inactivation state the GDP is bound to Gα (Gα-GDP). In signal transduction, first the GPCR gets activated by changing its conformation which resulted from binding of agonist/ligands to the extracellular region of GPCR. This activated GPCR further activate the inactive G protein to active G protein complex by dissociating the Gα from Gβγ. In active state the GTP is bound to Gα (Gα-GTP). Now free Gα and Gβγ have their own effectors (E1 and E2, respectively) to further transmit the signals and initiate unique intracellular signaling responses. Later, after the signal transduction, the Gα-GTPase activity hydrolyze the bound GTP (Gα-GTP) to GDP and Pi and inactivate the G protein complex by re-associating the Gα with Gβγ. In this state again GDP is bound to Gα (Gα-GDP) in the G protein complex. in this way the activation and inactivation cycle is completed.