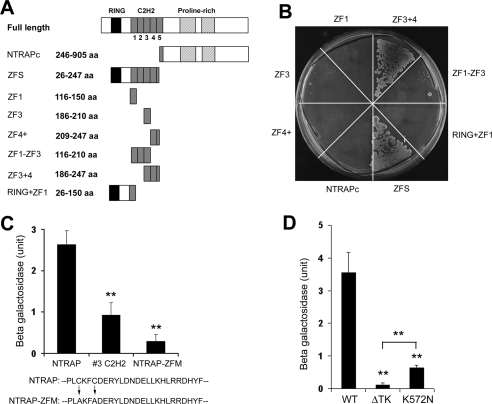
Figure 3.
Identification of interaction domains in NTRAP and TrkC. (A) Schematic representation of NTRAP mutants. (B) Yeast cells expressing the intracellular domain of TrkC fused to LexA and VP16-NTRAP mutants were streaked onto a histidine-free plate containing 10 mM 3-AT and grown at 30°C for 8 d. The number of grown yeast cells is an indicator of the interaction strength between TrkC and an NTRAP mutant. (C) Interaction of TrkC with NTRAP mutants. The #3 C2H2 construct is comprised of the VP16 activation domain and the third C2H2 zinc finger motif. The NTRAP-ZFM construct is comprised of the VP16 activation domain and an NTRAP mutant in which the two cysteine residues in the third C2H2 zinc finger have been changed to two alanine residues. The β-galactosidase activity in yeast two-hybrid assays reflects the interaction strength between two proteins. Error bars, SEs. **p < 0.01 by Student's t test; n = 3. (D) Interaction of NTRAP with wild-type TrkC (WT) or TrkC mutants (ΔTK and K572N). Error bars, SEs. **p < 0.01 by Student's t test; n = 3.