Abstract
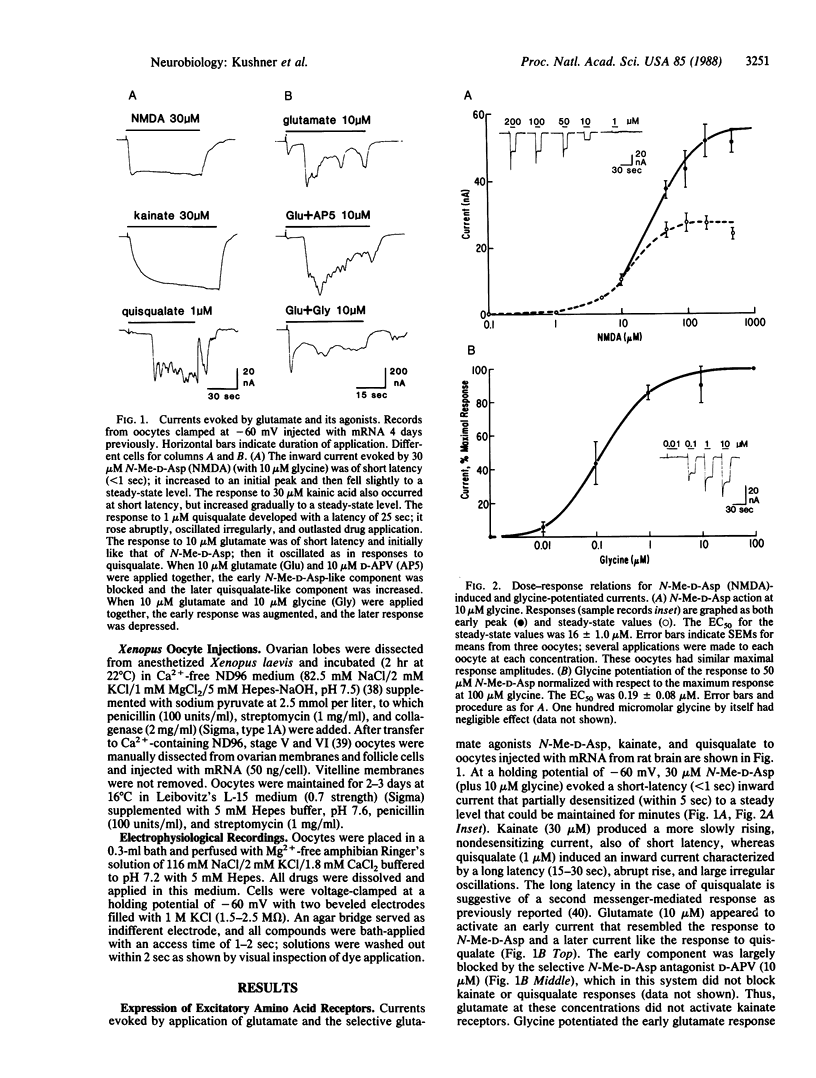
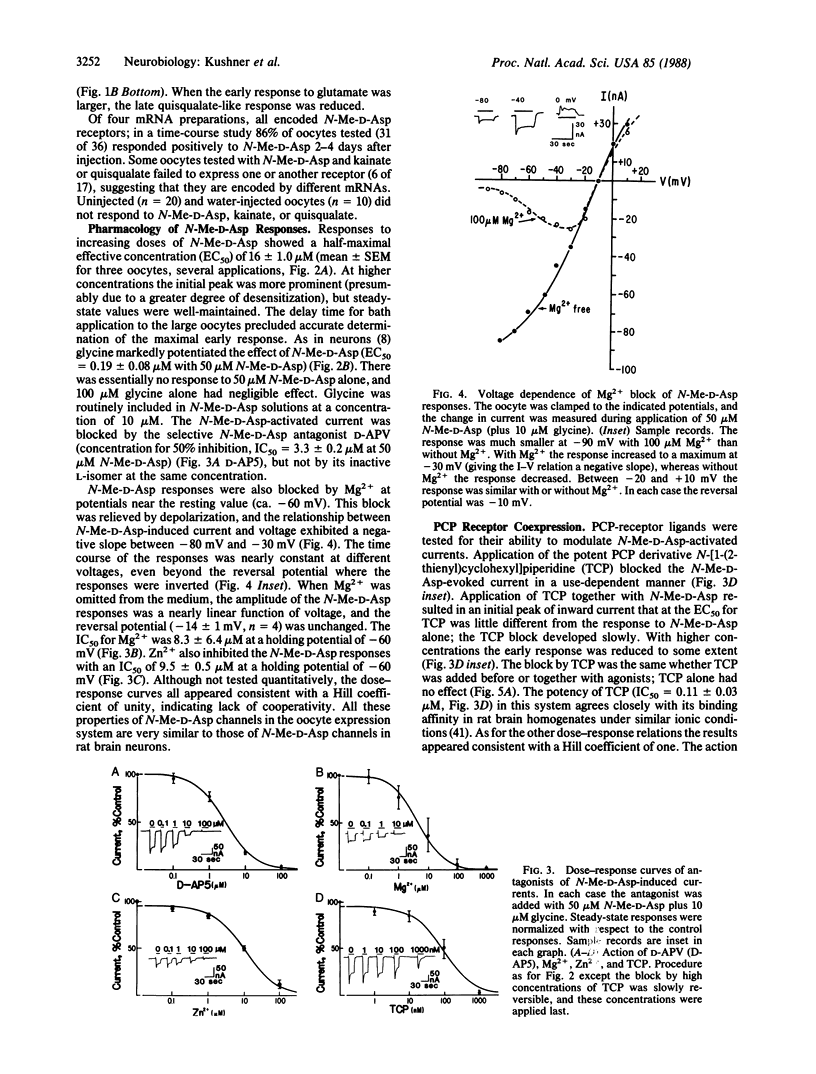
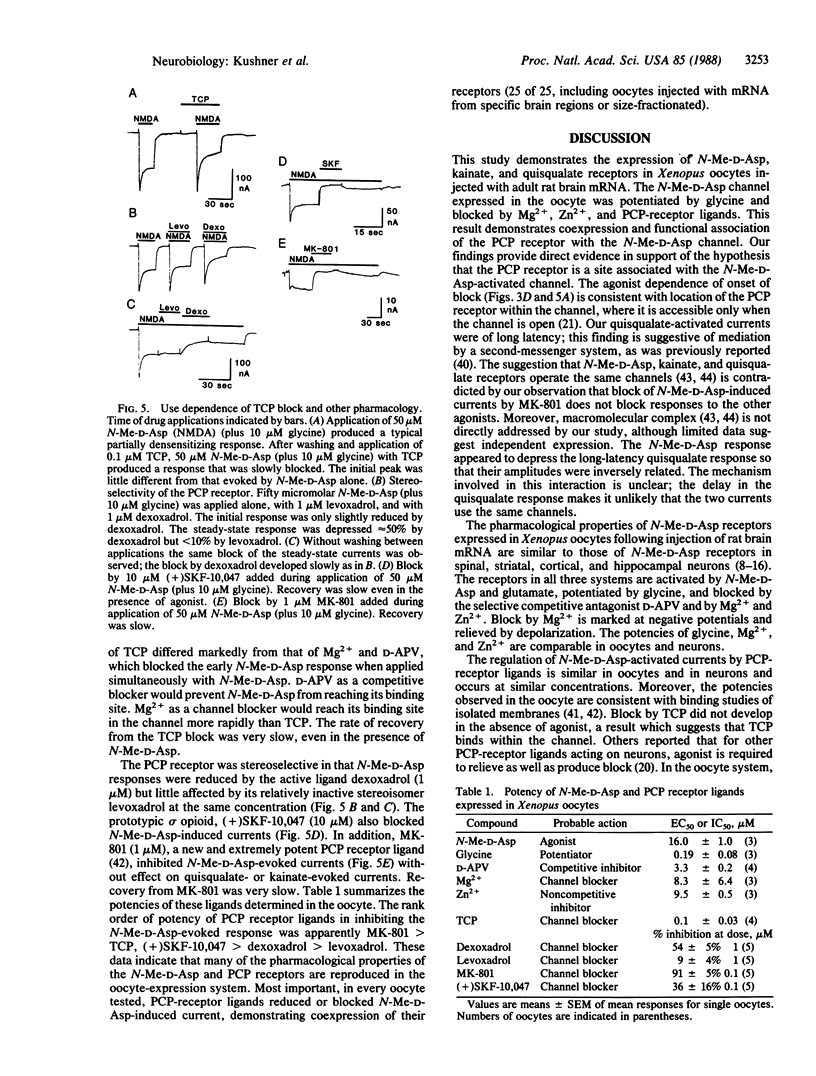
Recent evidence suggest that the N-methyl-D-aspartate (N-Me-D-Asp) channel is functionally and structurally associated with the phencyclidine (PCP) receptor, which mediates the psychotomimetic effects of PCP, sigma opioids, and dioxalanes. To investigate the relationship between N-Me-D-Asp and PCP receptors on a molecular level, we injected mRNA isolated from adult rat brain into Xenopus oocytes. In injected oocytes N-Me-D-Asp application (with glycine) evoked a partially desentizing inward current that was potentiated by glycine and blocked by D-(-)-amino-5-phosphonovaleric acid (D-APV), by Zn2+ and, in a voltage-dependent manner, by Mg2+. These results show that the distinguishing features of rat brain N-Me-D-Asp channels are reproduced in this translation system. In addition, kainic acid elicited a nondesensitizing inward current at short latency, and quisqualate elicited a delayed oscillatory inward current, presumably mediated by a second-messenger system. Responses to glutamate had both short-latency and delayed components. The PCP derivative N-[1-(2-thienyl)cyclohexyl]piperidine (TCP) blocked the N-Me-D-Asp-evoked current, and its potency was comparable to its binding affinity in rat brain membranes. Onset of block required the presence of antagonist. Antagonism was stereoselective in that the active ligand dexoxadrol was a more effective blocker than its relatively inactive stereoisomer levoxadrol. adrol. Other PCP receptor ligands, (+)SKF-10,047 and MK-801, also blocked. Potencies of compounds active at N-Me-D-Asp and PCP receptors in oocytes were comparable to those obtained previously in electrophysiological and binding assays on neural tissues. These results indicate the coexpression of neuronal PCP and N-Me-D-Asp receptors in Xenopus oocytes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anis N. A., Berry S. C., Burton N. R., Lodge D. The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. Br J Pharmacol. 1983 Jun;79(2):565–575. doi: 10.1111/j.1476-5381.1983.tb11031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard E. A., Miledi R., Sumikawa K. Translation of exogenous messenger RNA coding for nicotinic acetylcholine receptors produces functional receptors in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1982 May 22;215(1199):241–246. doi: 10.1098/rspb.1982.0040. [DOI] [PubMed] [Google Scholar]
- Berry S. C., Dawkins S. L., Lodge D. Comparison of sigma- and kappa-opiate receptor ligands as excitatory amino acid antagonists. Br J Pharmacol. 1984 Sep;83(1):179–185. doi: 10.1111/j.1476-5381.1984.tb10133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Usowicz M. M. Multiple-conductance channels activated by excitatory amino acids in cerebellar neurons. Nature. 1987 Feb 5;325(6104):525–528. doi: 10.1038/325525a0. [DOI] [PubMed] [Google Scholar]
- Dascal N., Snutch T. P., Lübbert H., Davidson N., Lester H. A. Expression and modulation of voltage-gated calcium channels after RNA injection in Xenopus oocytes. Science. 1986 Mar 7;231(4742):1147–1150. doi: 10.1126/science.2418503. [DOI] [PubMed] [Google Scholar]
- Dingledine R., Hynes M. A., King G. L. Involvement of N-methyl-D-aspartate receptors in epileptiform bursting in the rat hippocampal slice. J Physiol. 1986 Nov;380:175–189. doi: 10.1113/jphysiol.1986.sp016279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont J. N. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J Morphol. 1972 Feb;136(2):153–179. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Foster A. C., Fagg G. E. Acidic amino acid binding sites in mammalian neuronal membranes: their characteristics and relationship to synaptic receptors. Brain Res. 1984 May;319(2):103–164. doi: 10.1016/0165-0173(84)90020-1. [DOI] [PubMed] [Google Scholar]
- Gedamu L., Dixon G. H., Gurdon J. B. Studies of the injection of poly(A)+ protamine mRNA into Xenopus laevis oocytes. Exp Cell Res. 1978 Dec;117(2):325–334. doi: 10.1016/0014-4827(78)90146-5. [DOI] [PubMed] [Google Scholar]
- Ghysdael J., Hubert E., Trávnícek M., Bolognesi D. P., Burny A., Cleuter Y., Huez G., Kettmann R., Marbaix G., Portetelle D. Frog oocytes synthesize and completely process the precursor polypeptide to virion structural proteins after microinjection of avian myeloblastosis virus RNA. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3230–3234. doi: 10.1073/pnas.74.8.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen C. B., Miledi R., Parker I. Slowly inactivating potassium channels induced in Xenopus oocytes by messenger ribonucleic acid from Torpedo brain. J Physiol. 1984 Aug;353:231–248. doi: 10.1113/jphysiol.1984.sp015333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. W., Ganong A. H., Cotman C. W. Long-term potentiation in the hippocampus involves activation of N-methyl-D-aspartate receptors. Brain Res. 1984 Dec 3;323(1):132–137. doi: 10.1016/0006-8993(84)90275-0. [DOI] [PubMed] [Google Scholar]
- Honey C. R., Miljkovic Z., MacDonald J. F. Ketamine and phencyclidine cause a voltage-dependent block of responses to L-aspartic acid. Neurosci Lett. 1985 Oct 24;61(1-2):135–139. doi: 10.1016/0304-3940(85)90414-8. [DOI] [PubMed] [Google Scholar]
- Houamed K. M., Bilbe G., Smart T. G., Constanti A., Brown D. A., Barnard E. A., Richards B. M. Expression of functional GABA, glycine and glutamate receptors in Xenopus oocytes injected with rat brain mRNA. 1984 Jul 26-Aug 1Nature. 310(5975):318–321. doi: 10.1038/310318a0. [DOI] [PubMed] [Google Scholar]
- Jahr C. E., Stevens C. F. Glutamate activates multiple single channel conductances in hippocampal neurons. Nature. 1987 Feb 5;325(6104):522–525. doi: 10.1038/325522a0. [DOI] [PubMed] [Google Scholar]
- Jarvis M. F., Murphy D. E., Williams M. Quantitative autoradiographic localization of NMDA receptors in rat brain using [3H]CPP: comparison with [3H]TCP binding sites. Eur J Pharmacol. 1987 Sep 2;141(1):149–152. doi: 10.1016/0014-2999(87)90423-7. [DOI] [PubMed] [Google Scholar]
- Javitt D. C., Jotkowitz A., Sircar R., Zukin S. R. Non-competitive regulation of phencyclidine/sigma-receptors by the N-methyl-D-aspartate receptor antagonist D-(-)-2-amino-5-phosphonovaleric acid. Neurosci Lett. 1987 Jul 22;78(2):193–198. doi: 10.1016/0304-3940(87)90632-x. [DOI] [PubMed] [Google Scholar]
- Johnson J. W., Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987 Feb 5;325(6104):529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Lund T., Bravo R., Johansen H. R., Zeuthen J., Vuust J. Synthesis, processing, and secretion of rat immunoglobulin E made in Xenopus oocytes. FEBS Lett. 1986 Nov 24;208(2):369–372. doi: 10.1016/0014-5793(86)81051-1. [DOI] [PubMed] [Google Scholar]
- MacDonald J. F., Miljkovic Z., Pennefather P. Use-dependent block of excitatory amino acid currents in cultured neurons by ketamine. J Neurophysiol. 1987 Aug;58(2):251–266. doi: 10.1152/jn.1987.58.2.251. [DOI] [PubMed] [Google Scholar]
- Maragos W. F., Chu D. C., Greenamyre J. T., Penney J. B., Young A. B. High correlation between the localization of [3H]TCP binding and NMDA receptors. Eur J Pharmacol. 1986 Apr 9;123(1):173–174. doi: 10.1016/0014-2999(86)90703-x. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Guthrie P. B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984 May 17;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog Neurobiol. 1987;28(3):197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Parker I., Gundersen C. B., Miledi R. On the orientation of foreign neurotransmitter receptors in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1985 Dec 23;226(1244):263–269. doi: 10.1098/rspb.1985.0095. [DOI] [PubMed] [Google Scholar]
- Peters S., Koh J., Choi D. W. Zinc selectively blocks the action of N-methyl-D-aspartate on cortical neurons. Science. 1987 May 1;236(4801):589–593. doi: 10.1126/science.2883728. [DOI] [PubMed] [Google Scholar]
- Rauschecker J. P., Hahn S. Ketamine-xylazine anaesthesia blocks consolidation of ocular dominance changes in kitten visual cortex. Nature. 1987 Mar 12;326(6109):183–185. doi: 10.1038/326183a0. [DOI] [PubMed] [Google Scholar]
- Reynolds I. J., Murphy S. N., Miller R. J. 3H-labeled MK-801 binding to the excitatory amino acid receptor complex from rat brain is enhanced by glycine. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7744–7748. doi: 10.1073/pnas.84.21.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai Y., Kimura H., Okamoto K. Pharmacological characterization of serotonin receptor induced by rat brain messenger RNA in Xenopus oocytes. Brain Res. 1986 Jan 1;362(1):199–203. doi: 10.1016/0006-8993(86)91419-8. [DOI] [PubMed] [Google Scholar]
- Schofield P. R., Darlison M. G., Fujita N., Burt D. R., Stephenson F. A., Rodriguez H., Rhee L. M., Ramachandran J., Reale V., Glencorse T. A. Sequence and functional expression of the GABA A receptor shows a ligand-gated receptor super-family. Nature. 1987 Jul 16;328(6127):221–227. doi: 10.1038/328221a0. [DOI] [PubMed] [Google Scholar]
- Simon R. P., Swan J. H., Griffiths T., Meldrum B. S. Blockade of N-methyl-D-aspartate receptors may protect against ischemic damage in the brain. Science. 1984 Nov 16;226(4676):850–852. doi: 10.1126/science.6093256. [DOI] [PubMed] [Google Scholar]
- Sircar R., Rappaport M., Nichtenhauser R., Zukin S. R. The novel anticonvulsant MK-801: a potent and specific ligand of the brain phencyclidine/sigma-receptor. Brain Res. 1987 Dec 1;435(1-2):235–240. doi: 10.1016/0006-8993(87)91606-4. [DOI] [PubMed] [Google Scholar]
- Snell L. D., Johnson K. M. Antagonism of N-methyl-D-aspartate-induced transmitter release in the rat striatum by phencyclidine-like drugs and its relationship to turning behavior. J Pharmacol Exp Ther. 1985 Oct;235(1):50–57. [PubMed] [Google Scholar]
- Snell L. D., Johnson K. M. Characterization of the inhibition of excitatory amino acid-induced neurotransmitter release in the rat striatum by phencyclidine-like drugs. J Pharmacol Exp Ther. 1986 Sep;238(3):938–946. [PubMed] [Google Scholar]
- Sugiyama H., Ito I., Hirono C. A new type of glutamate receptor linked to inositol phospholipid metabolism. Nature. 1987 Feb 5;325(6104):531–533. doi: 10.1038/325531a0. [DOI] [PubMed] [Google Scholar]
- Sumikawa K., Houghton M., Emtage J. S., Richards B. M., Barnard E. A. Active multi-subunit ACh receptor assembled by translation of heterologous mRNA in Xenopus oocytes. Nature. 1981 Aug 27;292(5826):862–864. doi: 10.1038/292862a0. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Shine J., Chirgwin J., Pictet R., Tischer E., Rutter W. J., Goodman H. M. Rat insulin genes: construction of plasmids containing the coding sequences. Science. 1977 Jun 17;196(4296):1313–1319. doi: 10.1126/science.325648. [DOI] [PubMed] [Google Scholar]
- Verdoorn T. A., Kleckner N. W., Dingledine R. Rat brain N-methyl-D-aspartate receptors expressed in Xenopus oocytes. Science. 1987 Nov 20;238(4830):1114–1116. doi: 10.1126/science.2825347. [DOI] [PubMed] [Google Scholar]
- Westbrook G. L., Mayer M. L. Micromolar concentrations of Zn2+ antagonize NMDA and GABA responses of hippocampal neurons. Nature. 1987 Aug 13;328(6131):640–643. doi: 10.1038/328640a0. [DOI] [PubMed] [Google Scholar]
- Wigström H., Gustafsson B. A possible correlate of the postsynaptic condition for long-lasting potentiation in the guinea pig hippocampus in vitro. Neurosci Lett. 1984 Feb 24;44(3):327–332. doi: 10.1016/0304-3940(84)90044-2. [DOI] [PubMed] [Google Scholar]
- Zukin S. R., Zukin R. S. Specific [3H]phencyclidine binding in rat central nervous system. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5372–5376. doi: 10.1073/pnas.76.10.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]



