Figure 1.
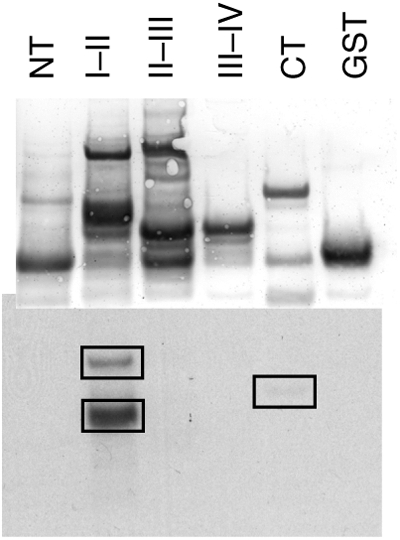
In vitro phosphorylation of the Na channel by CaMKII. Purified GST fusion proteins of the intracellular domains of NaV1.5 were phosphorylated in vitro with CaMKII in the presence of γ-32P-labelled ATP. Proteins were separated by SDS–PAGE and transferred to nitrocellulose. Total protein was visualized by Ponceau-S stain (top) and incorporated 32P was visualized by autoradiography (bottom). Phosphorylation occurred predominantly on the I–II linker, with a smaller amount occurring on the CT domain. NT, amino terminus; CT, carboxyl terminus. I–II, II-III, and III–IV denotes the I–II, II–III, and III–IV interdomain linkers, respectively.
