Table 3.
Substrate Scope.a
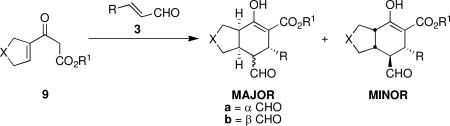 | ||||||
|---|---|---|---|---|---|---|
| entry | Product (major) | time (h) | yield (%)b | dr (maj:min)c | % ee (a)d | % ee (b)d |
| 1 | 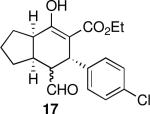 |
20 | 82 | 92:8 | 99 | 98 |
| 2 | 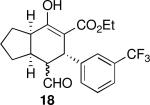 |
17 | 79 | 93:7 | 99 | 99 |
| 3 | 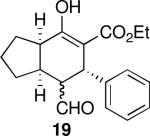 |
40 | 87 | 92:8 | 99 | 98 |
| 4 | 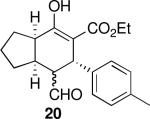 |
168 | 79 | 93:7 | 99 | 99 |
| 5 | 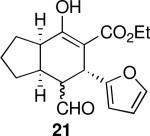 |
142 | 61 | 97:3 | 98 | 98 |
| 6 | 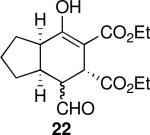 |
22 | 67 | 97:3 | 98 | 98 |
| 7 | 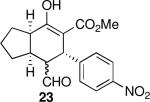 |
21 | 76 | 91:9 | 97 | 98 |
| 8 | 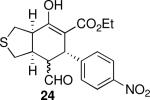 |
28 | 80 | 93:7 | 98 | 96 |
Reaction conditions: 3 (1 equiv), 9 (1 equiv), 1a (10 mol %), CF3CH2OH (0.3 M), rt.
Isolated yield.
Determined by 1H NMR of isolated products.
Determined by chiral HPLC.
