Fig. 6.
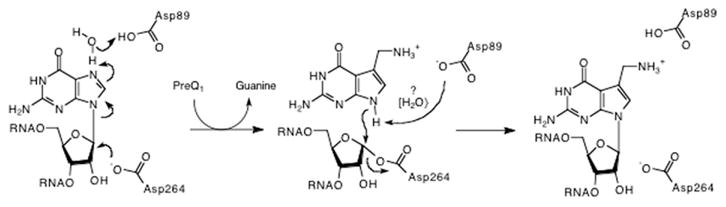
Chemical mechanism for the TGT reaction that is consistent with biochemical and structural data. Two active-site aspartate residues serve critical roles in catalysis by TGT. Aspartate 264 nucleophilically attacks the 1′-ribosyl carbon, resulting in a TGT–RNA covalent linkage and the displacement of the guanine base. It is hypothesized that aspartate 89 might be responsible for protonation of the guanine base and/or deprotonation of the incoming preQ1 (perhaps through the intermediacy of water). While guanine is shown being protonated at N7, protonation could be directly at N9.
