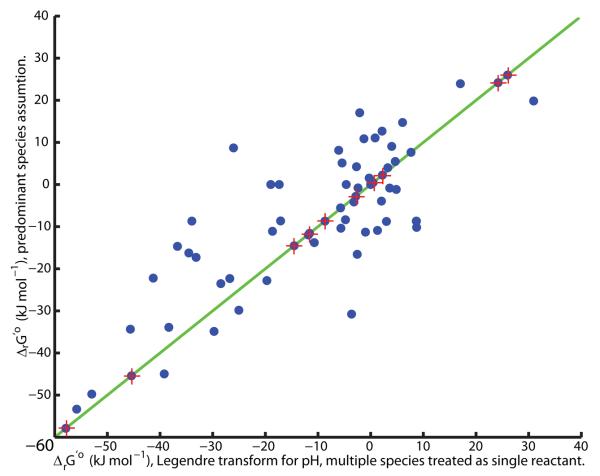
Figure 3. The importance of a true Legendre Transform.
When cytoplasmic pH is held constant by buffering, a Legendre transform of metabolite species standard Gibbs energy of formation defines a standard transformed Gibbs energy of formation for each metabolite species. Reactant standard transformed Gibbs energy of formation, and therefore reaction standard transformed Gibbs energy, depends non-additively on the standard transformed metabolite species Gibbs energy of formation. Due to this non-additivity, when reactants are present as multiple species, differing in their state of protonation, it is erroneous to replace transformation of metabolite species standard Gibbs energy of formation, with adjustment to the hydrogen ion standard Gibbs energy of formation, when calculating reaction standard transformed Gibbs energy. The latter gives rise to an erroneous estimate of standard ‘transformed’ reaction Gibbs energy, as illustrated above for the reactions in the central metabolic E. coli model [61] at pH 7.7, zero ionic strength, atmospheric pressure and temperature 298.15K. The exception is when each reactant involved in a reaction is present as only one metabolite species. In this case both approaches agree (stars on the diagonal).