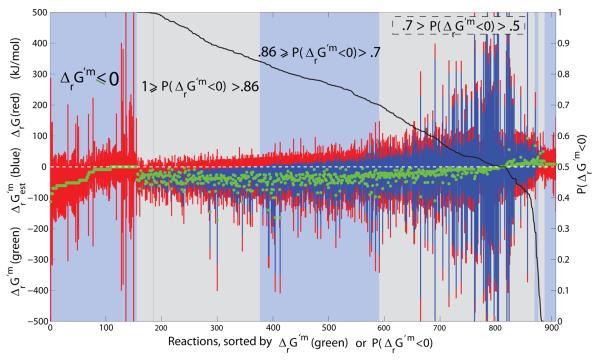
Figure 4. Qualitatively forward, quantitatively reverse reactions.
The reactions that are qualitatively assigned to be forward in iAF1260, yet quantitatively reversible, using at least one group contribution estimate of reactant standard transformed Gibbs energy of formation. The feasible range of ΔrG′ and are given as red and blue bars respectively. Far left are transport reactions with negative or zero physiological standard transformed reaction Gibbs energy, ΔrG′m ≤ 0, but reversible depending on concentration of reactants. Reactions with uncertainty due to estimation of standard transformed reaction Gibbs energy are rank ordered by decreasing probability that physiological standard transformed reaction Gibbs energy is negative. In mathematical notation this probability is represented by the symbol P(ΔrG′m < 0), as defined in Eq. 3, and used in situabove to denote the intervals as follows: Reactions with P(ΔrG′m < 0) > 0.7 were assumed to be irreversible in the forward direction, and reactions with P(ΔrG′m < 0) < 0.3 or ΔrG′m > 0, were assumed to be irreversible in the reverse direction. See Figure 12 for a detailed illustration of the latter reactions. Reactions with 0.7 ≥ P(ΔrG′m < 0) > 0.3 were allowed to be quantitatively reversible in lieu of the large uncertainty in estimation of standard transformed reaction Gibbs energy.