Figure 5. Feeding peptide to load a bolus of MHC class I molecules increases the amounts of MHC class I and Bap31 colocalized with the ERGIC marker, ERGIC-53.
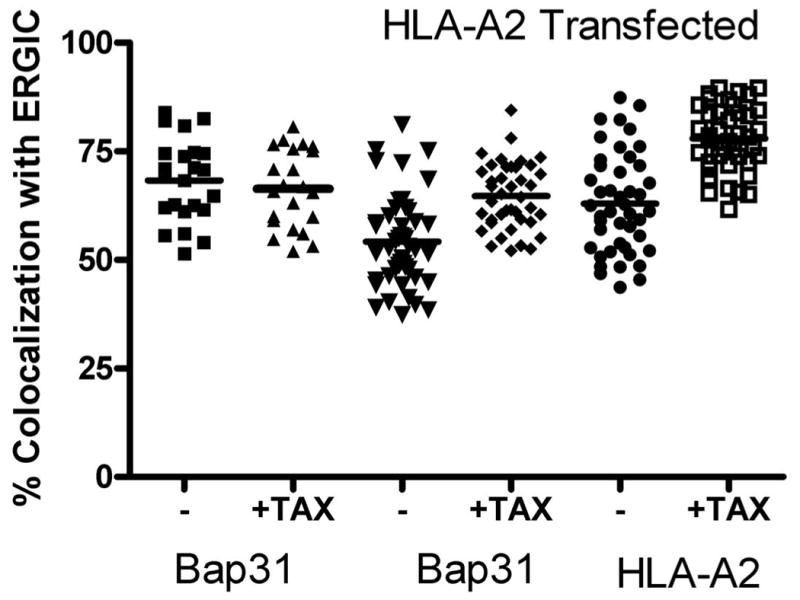
A) Cells were transfected with HLA-A2-YFP. Approximately 20 hours later the cells were fed a high-affinity peptide, TAX (LLFGYPVYV) for 30 minutes. The cells were then permeabilized and endogenous ERGIC-53 and Bap31 were labeled with the appropriate antibodies. Feeding peptide to cells which express HLA-A2-YFP significantly increased the fraction of ERGIC marker colocalized with Bap31 (p<0.001).
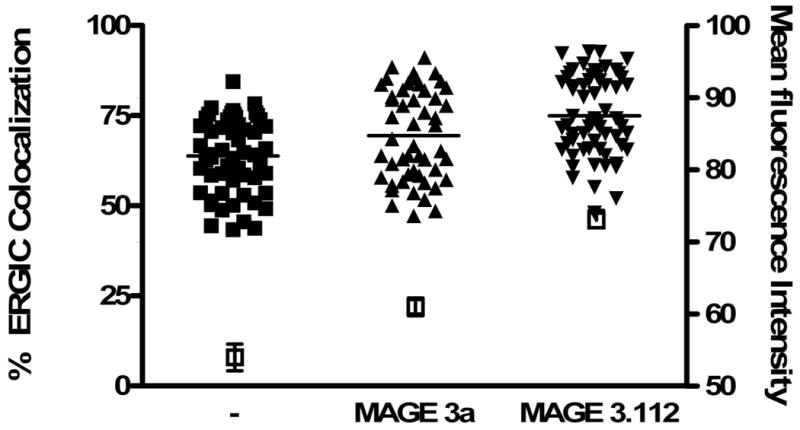
B) Cells were fed peptides predicted to differ by 10-fold in their affinity for HLA-A68, an endogenous HLA molecule of HeLa cells: MAGE3.112, which binds HLA-A-68 with high affinity (12) and MAGE 3a, which is predicted to bind with ∼10-fold lower affinity (13,14). Consistent with this prediction MAGE 3a rescued fewer empty MHC class I molecules from acid-stripped cells (15) than did MAGE 3.112. This was detected by flow cytometry in terms of binding of fluorescent KE-2 IgG. The open symbols and right-hand axis represent mean channel fluorescence intensity +/- 95% confidence interval for control cells, treated with weak acid, pH 3.2, for 90 s, then washed and incubated in buffer at 4°C for 1 hour followed by incubation in buffer at 37°C for 1 hour, and for cells so treated, but incubated with 12μM peptide as indicated. For colocalization studies, peptide was fed for 30 min, the cells were fixed, permeabilized and then stained for Bap31 as described in ‘Materials and Methods’. Both peptides significantly increased the extent of ERGIC colocalization with Bap31 (p<0.05 for MAGE3a and p<0.001 for MAGE 3.112).
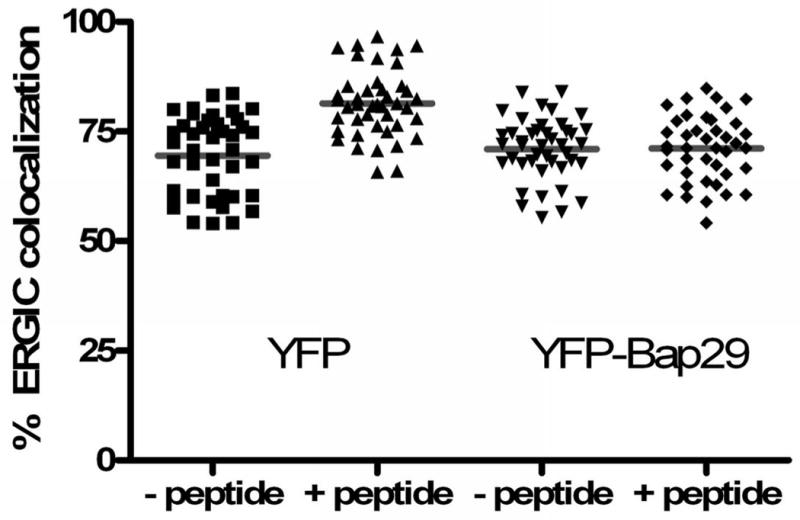
C) Expression of Bap29 blocks the peptide-dependent forward traffic of Bap31. Cells were transfected with either free YFP or with YFP-Bap29. Feeding peptide to YFP-expressing control cells had no effect on forward traffic of Bap31 induced by feeding MAGE 3.112 (left bars), but forward traffic was blocked in cells expressing Bap29-YFP (right bars).
