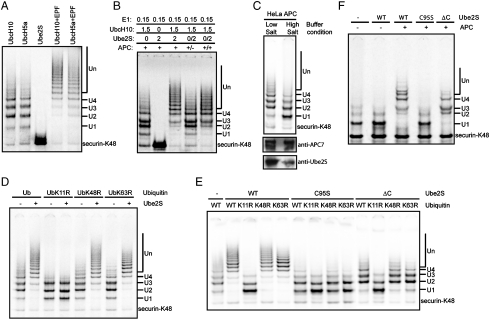
Fig. 4.
UBE2S assembles K11 linked ubiquitin chains on APC substrates. (A) Cooperativity between UbcH10 or UbcH5a and UBE2S. 35S-labeled securin-K48 were ubiquitinated in the presence of different combinations and concentrations of E2s, as indicated. (B) UBE2S’s ubiquitin-chain extension activity is APC-dependent. 35S labeled securin-K48 was ubiquitinated in the presence of different combinations and concentrations of E2s as indicated. The Lane (+/-) designates a reaction, where substrate was ubiquitinated first with UbcH10 for 1 h, then the products were separated from APC and ubiquitinated with UBE2S for another 1 h. (C) UBE2S is required for synthesizing long ubiquitin chains. 35S-labeled securin-K48 were ubiquitinated with UbcH10 and APC that were purified under different conditions. Purified APC samples were immuno-blotted with anti-APC7 and anti-UBE2S antibodies. (D) UBE2S makes K11 specific ubiquitin chains. 35S labeled securin-K48 were ubiquitinated in the absence (-) or presence (+) of UBE2S and UbcH10 with different ubiquitin mutants. (E) UBE2S’s ubiquitin-chain extension activity requires its catalytic residue and the C-terminal domain. 35S-labeled securin-K48 were ubiquitinated in the absence (-) or presence of UBE2S mutants and UbcH10 with different ubiquitin mutants. (F) UBE2S extends ubiquitin chains on pre-ubiquitinated substrate. 35S-labeled securin-K48 were first ubiquitinated with GST-UbcH5a. After removing APC and GST-UbcH5a by immunodepletion, the products were used as substrate for a second ubiquitination reaction with (+) or without (-) APC in presence of a different UBE2S mutant. All the ubiquitination reactions were analyzed by autoradiography.