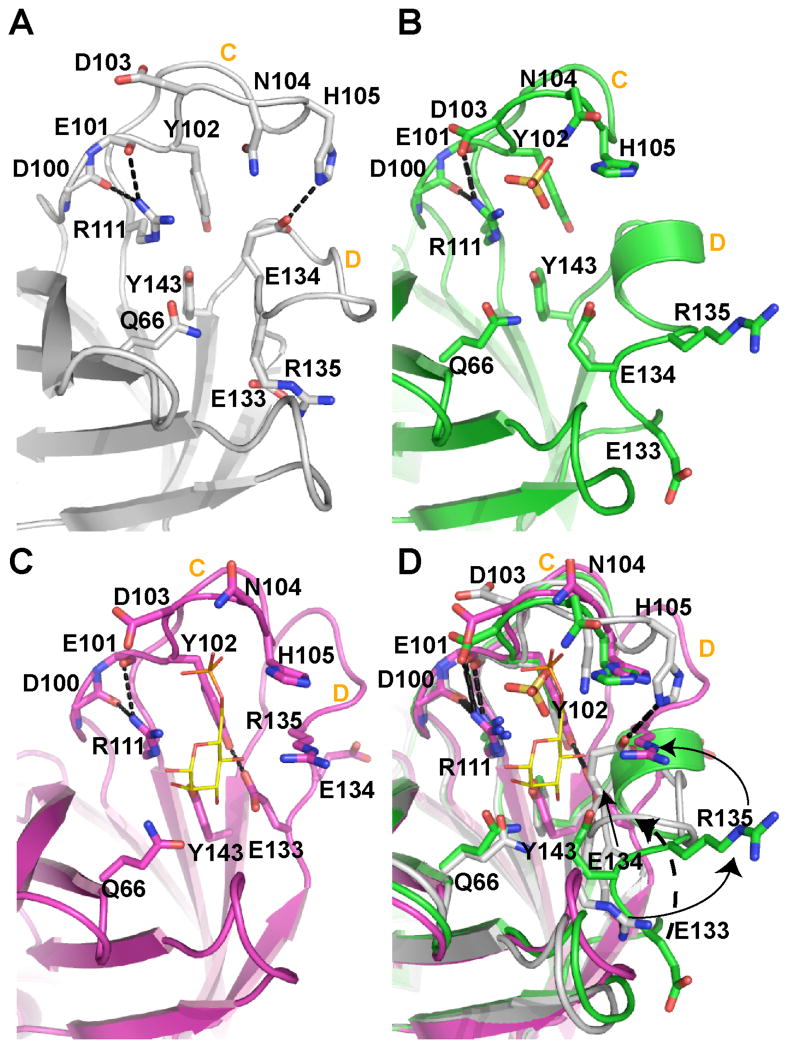
FIGURE 6. Close-up view of the binding site unoccupied, partially occupied, or fully bound to ligand.
(A) In the complete absence of ligand, loop C is elongated to form a β-finger (sCD-MPR, 2RL7). (B) However, loop C contracts in the presence of a sulfate ion (yellow ball-and-stick) (EQKM/sulfate, 3K42). The presence of sulfate also results in the movement of loop D out of the binding pocket and the reordering of loop D to form an intermediate α-helix. (C) The occupation of both the phosphate and mannose binding regions of the receptor (sCD-MPR, 2RL8) transitions the residues of loop D to the side of the binding pocket. (D) Superimposition of all three states of the binding pocket. Residues are labeled as well as loops C and D in all four panels.