Abstract
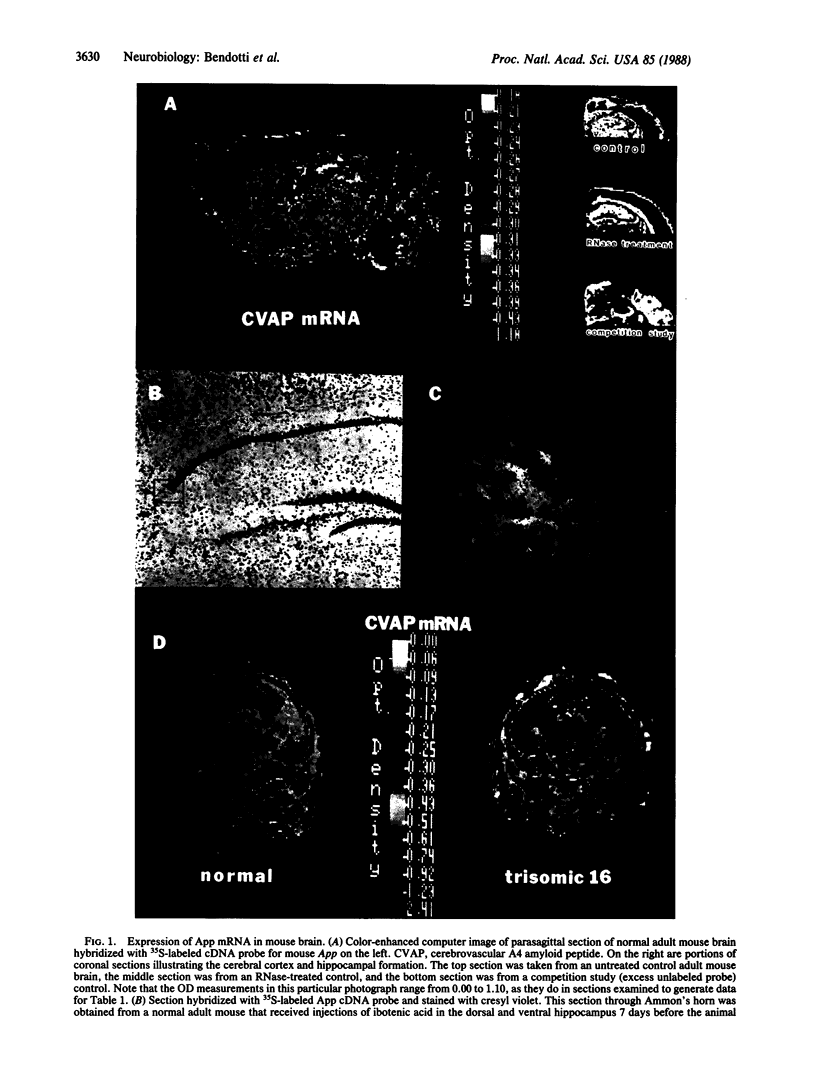
Amyloid precursor protein mRNA was localized in frozen sections from normal and experimentally lesioned adult mouse brain and from normal and aneuploid fetal mouse brain by in situ hybridization with a 35S-labeled mouse cDNA probe. The highest levels of hybridization in adult brain were associated with neurons, primarily in telencephalic structures. The dense labeling associated with hippocampal pyramidal cells was reduced significantly when the cells were eliminated by injection of the neurotoxin ibotenic acid but was not affected when electrolytic lesions were placed in the medial septum. Since the gene encoding amyloid precursor protein has been localized to mouse chromosome 16, we also examined the expression of this gene in the brains of mouse embryos with trisomy 16 and trisomy 19 at 15 days of gestation. RNA gel blot analysis and in situ hybridization showed a marked increase in amyloid precursor protein mRNA in the trisomy 16 mouse head and brain when compared with euploid littermates or with trisomy 19 mice.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahmanyar S., Higgins G. A., Goldgaber D., Lewis D. A., Morrison J. H., Wilson M. C., Shankar S. K., Gajdusek D. C. Localization of amyloid beta protein messenger RNA in brains from patients with Alzheimer's disease. Science. 1987 Jul 3;237(4810):77–80. doi: 10.1126/science.3299701. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consolo S., Wang J. X., Fusi R., Vinci R., Forloni G., Ladinsky H. In vitro and in vivo evidence for the existence of presynaptic muscarinic cholinergic receptors in the rat hippocampus. Brain Res. 1984 Aug 20;309(1):147–151. doi: 10.1016/0006-8993(84)91019-9. [DOI] [PubMed] [Google Scholar]
- Cox D. R., Epstein C. J. Comparative gene mapping of human chromosome 21 and mouse chromosome 16. Ann N Y Acad Sci. 1985;450:169–177. doi: 10.1111/j.1749-6632.1985.tb21491.x. [DOI] [PubMed] [Google Scholar]
- Coyle J. T., Oster-Granite M. L., Gearhart J. D. The neurobiologic consequences of Down syndrome. Brain Res Bull. 1986 Jun;16(6):773–787. doi: 10.1016/0361-9230(86)90074-2. [DOI] [PubMed] [Google Scholar]
- Coyle J. T., Price D. L., DeLong M. R. Alzheimer's disease: a disorder of cortical cholinergic innervation. Science. 1983 Mar 11;219(4589):1184–1190. doi: 10.1126/science.6338589. [DOI] [PubMed] [Google Scholar]
- Delabar J. M., Goldgaber D., Lamour Y., Nicole A., Huret J. L., de Grouchy J., Brown P., Gajdusek D. C., Sinet P. M. Beta amyloid gene duplication in Alzheimer's disease and karyotypically normal Down syndrome. Science. 1987 Mar 13;235(4794):1390–1392. doi: 10.1126/science.2950593. [DOI] [PubMed] [Google Scholar]
- Eicher E. M., Washburn L. L. Assignment of genes to regions of mouse chromosomes. Proc Natl Acad Sci U S A. 1978 Feb;75(2):946–950. doi: 10.1073/pnas.75.2.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Gearhart J. D., Davisson M. T., Oster-Granite M. L. Autosomal aneuploidy in mice: generation and developmental consequences. Brain Res Bull. 1986 Jun;16(6):789–801. doi: 10.1016/0361-9230(86)90075-4. [DOI] [PubMed] [Google Scholar]
- Gearhart J. D., Oster-Granite M. L., Reeves R. H., Coyle J. T. Developmental consequences of autosomal aneuploidy in mammals. Dev Genet. 1987;8(4):249–265. doi: 10.1002/dvg.1020080408. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984 May 16;120(3):885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Goldgaber D., Lerman M. I., McBride O. W., Saffiotti U., Gajdusek D. C. Characterization and chromosomal localization of a cDNA encoding brain amyloid of Alzheimer's disease. Science. 1987 Feb 20;235(4791):877–880. doi: 10.1126/science.3810169. [DOI] [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Kornguth S. E., Bersu E. T., Auerbach R., Sobkowicz H. M., Schutta H. S., Scott G. L. Trisomy 16 mice: neural, morphological, and immunological studies. Ann N Y Acad Sci. 1986;477:160–178. doi: 10.1111/j.1749-6632.1986.tb40331.x. [DOI] [PubMed] [Google Scholar]
- Lovett M., Goldgaber D., Ashley P., Cox D. R., Gajdusek D. C., Epstein C. J. The mouse homolog of the human amyloid beta protein (AD-AP) gene is located on the distal end of mouse chromosome 16: further extension of the homology between human chromosome 21 and mouse chromosome 16. Biochem Biophys Res Commun. 1987 Apr 29;144(2):1069–1075. doi: 10.1016/s0006-291x(87)80073-6. [DOI] [PubMed] [Google Scholar]
- Masters C. L., Simms G., Weinman N. A., Multhaup G., McDonald B. L., Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster-Granite M. L. The neurobiologic consequences of autosomal trisomy in mice and men. Brain Res Bull. 1986 Jun;16(6):767–771. doi: 10.1016/0361-9230(86)90073-0. [DOI] [PubMed] [Google Scholar]
- Reeves R. H., Gearhart J. D., Littlefield J. W. Genetic basis for a mouse model of Down syndrome. Brain Res Bull. 1986 Jun;16(6):803–814. doi: 10.1016/0361-9230(86)90076-6. [DOI] [PubMed] [Google Scholar]
- Reeves R. H., Robakis N. K., Oster-Granite M. L., Wisniewski H. M., Coyle J. T., Gearhart J. D. Genetic linkage in the mouse of genes involved in Down syndrome and Alzheimer's disease in man. Brain Res. 1987 Sep;388(3):215–221. doi: 10.1016/0169-328x(87)90028-3. [DOI] [PubMed] [Google Scholar]
- Robakis N. K., Ramakrishna N., Wolfe G., Wisniewski H. M. Molecular cloning and characterization of a cDNA encoding the cerebrovascular and the neuritic plaque amyloid peptides. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4190–4194. doi: 10.1073/pnas.84.12.4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltarelli M. D., Forloni G. L., Oster-Granite M. L., Gearhart J. D., Coyle J. T. Neurochemical characterization of embryonic brain development in trisomy 19 (Ts19) mice: implications of selective deficits observed for abnormal neural development in aneuploidy. Dev Genet. 1987;8(4):267–279. doi: 10.1002/dvg.1020080409. [DOI] [PubMed] [Google Scholar]
- Scott B. S., Becker L. E., Petit T. L. Neurobiology of Down's syndrome. Prog Neurobiol. 1983;21(3):199–237. doi: 10.1016/0301-0082(83)90002-3. [DOI] [PubMed] [Google Scholar]
- Tanzi R. E., Gusella J. F., Watkins P. C., Bruns G. A., St George-Hyslop P., Van Keuren M. L., Patterson D., Pagan S., Kurnit D. M., Neve R. L. Amyloid beta protein gene: cDNA, mRNA distribution, and genetic linkage near the Alzheimer locus. Science. 1987 Feb 20;235(4791):880–884. doi: 10.1126/science.2949367. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski K. E., Wisniewski H. M., Wen G. Y. Occurrence of neuropathological changes and dementia of Alzheimer's disease in Down's syndrome. Ann Neurol. 1985 Mar;17(3):278–282. doi: 10.1002/ana.410170310. [DOI] [PubMed] [Google Scholar]
- Young W. S., 3rd Periventricular hypothalamic cells in the rat brain contain insulin mRNA. Neuropeptides. 1986 Aug-Sep;8(2):93–97. doi: 10.1016/0143-4179(86)90035-1. [DOI] [PubMed] [Google Scholar]