Abstract
Our aim was to understand the information from differential two-sugar excretion (2-SE) in measuring intestinal permeability. In a crossover study in 12 healthy volunteers, we compared urinary excretion ratios of lactulose (L) to mannitol [(M) LMR] after ingestion in liquid formulation (LF) or in delayed-release, methacrylate-coated capsules (CAP). Both formulations were radiolabeled. Urine was collected every 2 hours from 0–8h, and from 8–24h. Two hours after LF, gastric residual was 15.9 ± 6.2 % (SEM), and the percentage in colon was 49.6 ± 7.8 %; in 11/12 participants, liquid had entered colon within 2h. Average CAP arrival time in colon was 5.16 ± 0.46h (mode 6 h). After LF, mannitol was extensively absorbed in the first 8h; lactulose absorption was low thoughout the 24h. After the LF, the LMR (geometric mean, 95% CI/hour) in the 0–2h urine was 0.08 [0.05, 0.11]), which was lower than in 8–24h urine (0.32,[0.16, 0.46]; p<0.05). Urine LMRs at 8–24h were similar after LF or CAP. We concluded that, after LF, sugar excretion in 0–2h urine may reflect both SI and colon permeability. Colonic permeability is reflected by urine sugar excretion between 6 and 24h. CAP delivery reduces mannitol excreted at 0–6h, compared to LF. The 0 to 5 or 6h 2-SE urine likely reflects both SI and colon permeability; the higher LMR in the 8–24h urine relative to 0–2h urine should be interpreted with caution and does not mean that colon is more permeable than SI.
Keywords: barrier, irritable bowel, inflammation, LMR
Differential absorption and excretion of molecular probes provide evidence of altered permeability of the intestine in gastrointestinal diseases including celiac disease, Crohn’s disease and irritable bowel syndrome (IBS). Greater paracellular permeability could facilitate passage of luminal antigens, leading to local mucosal immune responses and resulting in inflammation (1) or stimulation of bowel dysfunction or visceral pain.
Some highly sensitive methods for measuring intestinal permeability are too invasive to be used routinely. Ussing chamber techniques, requiring multiple intestinal mucosal biopsies at different levels of the gut, are too invasive to be used in large scale research on humans or for clinical diagnosis (2). Measurements using a single molecule (such as 51Cr EDTA) are potentially affected by interindividual differences not related to permeability (e.g. transit or urinary excretion). Thus, human intestinal permeability has been measured by urinary excretion of two probes of different sizes but similar transit and uptake processes, and calculating the excretion ratio of a monosaccharide and a disaccharide such as mannitol and lactulose respectively (3). The amounts of lactulose and mannitol in the normal diet are negligible to trace. Other molecules are used, including sucralose. This is an artificial sweetener, and dietary intake may interfere with its use in measurement of intestinal permeability.
A subgroup of IBS patients has increased permeability (reviewed in reference 4). In one study (5), the non-post-infectious (PI) IBS patients had greater permeability compared to PI-IBS; this observation appeared to contradict the hypothesis that a presumably inflamed mucosa in PI-IBS is more permeable, as observed in other conditions such as Crohn’s disease. In another study, there was no statistically significant difference between PI-IBS and controls using the pre-specified cut-off for increased permeability [lactulose:mannitol ratio (LMR) >0.025], although the proportion of controls with LMR <0.02 was higher than the proportion of IBS patients (6). Assessment of permeability may discriminate IBS from organic disease (7), The significance of LMR and its performance characteristics require further study.
In a commonly used method, sugars are ingested in water and, after fasting for 2 hours, urine is collected over a 24-hour period and the LMR from the 0–6 hour urine collection is used to estimate small intestinal (SI) permeability (8). In other studies, urine at 0–3, 3–5, and 5–24 hours is used to reflect permeability of the proximal SI, distal SI, and colon respectively (9–11). However, radioscintigraphic transit studies in health and disease indicate arrival in the colon of liquid within 4 hours (average) and <2 hours even when ingested with a mixed meal (12,13). It is, therefore, unclear which timed urine collections during the first 6 hours after ingestion reflect SI or colonic absorption of probe molecules..
Our specific aim was to compare urinary excretion of lactulose and mannitol after liquid versus delayed-release capsule formulations over 24 hours in order to understand the urine excretion profiles of each sugar, concordance of information from individual sugar urine excretion with the LMR, and to assess the validity of the LMR to characterize intestinal permeability in healthy humans.
METHODS
Design and Participants
In twelve healthy volunteers (6 male and 6 female), we performed a crossover study of urine L:M excretion ratio after liquid versus delayed-release capsule formulations; the studies were separated by an average of 7 days. The methacrylate-coated capsules dissolve in the close to neutral pH in the terminal ileum, and they serve to deliver the sugars to the distal ileum or proximal colon. The order of administrations was randomized and blinded from the laboratory personnel conducting the urine assays. Exclusion criteria were: use of tobacco products within the prior 6 months or of NSAIDs within the prior week.
Sugars and Radioisotopes
The sugars, mannitol 1 g and lactulose 5 g, were ingested either with 99mTc-DTPA in 180 mL water or in methacrylate-coated gelatin capsules on to which 111In-DTPA in 100 µl was applied by impregnating cellulose filter paper, attaching it to the gelatin capsule. The capsule was then dipped into the pH-sensitive polymer, methacrylate, which dissolves in the more neutral pH in the distal ileum. This method has been used extensively to deliver radioisotope to the colon for transit studies in an estimated 20,000 participants in the research laboratory or the clinical Nuclear Medicine Laboratory at Mayo Clinic over the last 20 years, with >90% of studies. We had validated that a single coating of methacrylate was necessary to ensure ileocolonic dissolution and that more coatings would result in failure of capsule dissolution (14). The isotope facilitated identification of the time of arrival of the capsule formulation in the cecum. Similarly, the emptying of 99mTc-DTPA from the stomach and its arrival in the right colon were documented by external scintigraphy.
Urine Collections and Abdominal Imaging
After oral ingestion of the sugars in water or capsules, urine was collected in 2-hour aliquots for the first 8 hours, followed by a collection from 8 to 24 hours to estimate the time of detection of the total content of each sugar in each aliquot, as well as the ratio of the excretion of the two sugars. The volume of each collection was measured and the urine aliquot stored at −20 degrees Celsius until it was thawed for analysis.
Gamma camera images were also obtained every 2 hours during the first 8 hours to estimate when the isotope first reached the right colon, identified by markers placed over the right anterior superior iliac spine. The amount of radiolabel in the liquid formulation remaining in the stomach and the amount located in the colon at 2 hours after ingestion were quantitated using a variable region of interest program. Since each participant also had an anatomical image of the colon from the study using liquid radioisotope, the two images (with solid and liquid radiolabel) could be superimposed to determine the time when the radiolabel from the capsule was unequivocally in the colon.
Measurement of Urine Sugars
This is described in detail in the Appendix. Urine sugar concentrations were measured by liquid chromatography-tandem mass spectrometry using an adapted approach to that recently reported in the literature (15).
Independently, the samples were also measured by an outside reference laboratory (Genova Diagnostics, Asheville, NC) using enzyme assays for each sugar (16–19).
Data and Statistical Analysis
The profiles of excretion of the two sugars after the two administrations were plotted and compared to qualitatively assess the excretion profiles. All data are presented as median and interquartile range, unless otherwise stated. The timing of arrival of the isotope in the colon was noted in each study. The statistical power of the study is included in the Appendix.
Intestinal permeability was measured using the lactulose and mannitol excretion test in all healthy controls. The results were expressed as the ratio of excretion of the ingested dose of lactulose and mannitol in urine. The total amount of lactulose was 5 g and mannitol was 1 g:
Excretion at time t = [concentration of sugar (mg/mL) at t ]* total urine volume (mL) at t
The cumulative excretion up to time t (mg) is then expressed as a percent (%) cumulative excretion by dividing by the total amount of sugar ingested (mg) and multiplying by 100:
% Cumulative Excretion= 100* (Σt excretiont) / total ingested
The differences in (percentage) cumulative excretion for lactulose compared to mannitol were then assessed using a repeated measures analysis of variance (AOV) with terms for type of vehicle used to deliver the sugars (liquid vs. capsule) and type of sugar as the repeated factors. This analysis was done separately at each time point and the alpha level for significance of main effects and interactions (vehicle by sugar type) in each analysis was set at 0.01 to adjust for the 5 time points. In addition, for each type of sugar separately, a repeated measures AOV was used with time and type of delivery as the repeat factors. This approach aimed to assess the profile (time course) in cumulative excretion for each type of sugar separately and whether these profiles differed by type of delivery (liquid vs. capsule). A term to check for possible order effects (i.e. having the capsule administration first or the liquid formulation first) was also included in each of these analyses.
Bland-Altman plots were used to compare the results of the LC-MS assay and the commercial assay. The profile and cumulative excretion of each sugar were plotted after liquid formulation or capsule administration.
The LMR excretion was computed for each subject’s study for each timed collection:
LMR = Excretion Lactulose (mg/hr) / Excretion Mannitol(mg/hr)
Due to the positively skewed distribution of these ratios, they were first transformed to log scale and a repeated measures AOV with time and type of delivery (capsule vs. liquid) as the repeat factors, along with a type by time interaction term included in the model. The least-squares means (and 95% CIs) were then transformed back to the original ratio scale yielding geometric mean ratios for plotting and summarization of the ratio data.
Statistical power is addressed in the Appendix.
RESULTS
Accuracy of LC-MS Method Relative to Commercial Assay
Linearity, precision and recovery of the sugars in urine were excellent and are reported in the Appendix. The correlation coefficient between the two assays for mannitol was 0.977 (p<0.001) and for lactulose 0.662 (p<0.001).
In order to assess the accuracy and validity of the measurements by HPLC-MS/MS, 80% of the samples were sent to a commercial laboratory (Genova Diagnostics, Asheville, NC). Comparisons of sugar excretion showed that the LC-MS/MS method trended with the commercial enzyme assay-based method for both analytes and for both administration methods (Figure 1). The LC-MS/MS method did not show saturation at later time points in the urine collections; this saturation was observed in several samples at the later urine collections with the commercial assay (data not shown). Accuracy was assessed by Bland-Altman analysis using the information from studies conducted with either the liquid or capsule formulations (Figure 2). For the 0–2 hour collections, we included only the observations obtained with the liquid formulation. For the 8–24 hour collections, the data in Figure 2 provide all information obtained by liquid or capsule formulations. As expected, the variation is somewhat higher relative to median concentration for lactulose than for mannitol, since the excretion of lactulose is about 10% that of mannitol.
Figure 1.
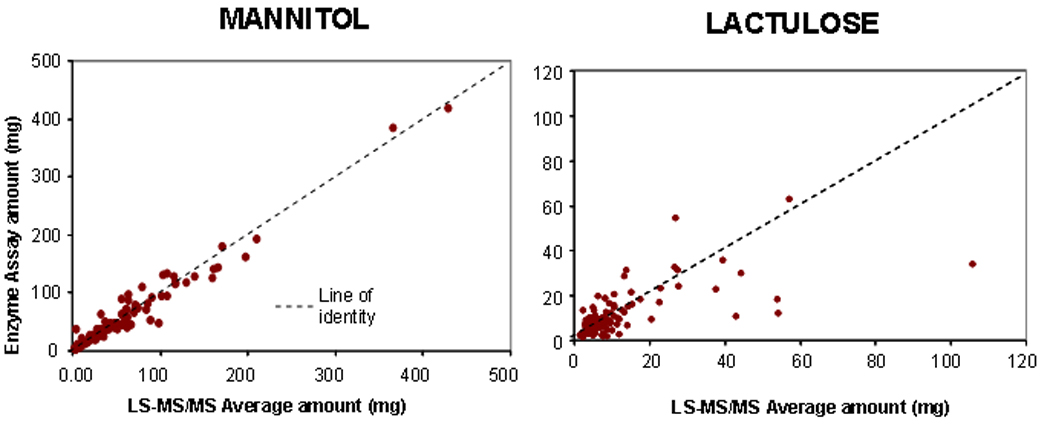
Accuracy of LS/MS-MS measurements of urinary lactulose and mannitol in comparison to the (commercial) enzyme-based assay. Note most of the data fall close to the line of identity. Correlation coefficient for mannitol and lactulose are respectively 0.977 and 0.662 (both p<0.001)
Figure 2.
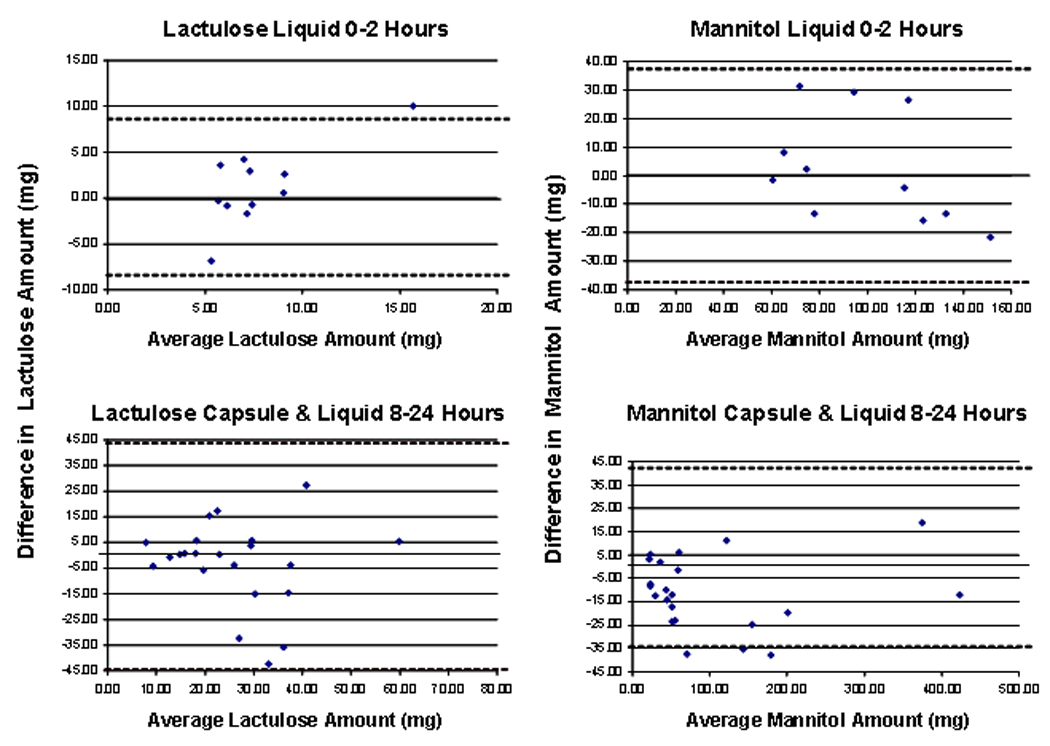
Bland Altman plots demonstrating the delta enzyme assay minus average of 2 estimates LC/MS-MS assay on the Y axis and the average of the enzyme assay and the mean of 2 estimates by LC/MS-MS on the x axis.
Time of Gastric Emptying and Appearance of Isotope in Right Colon
Two hours after liquid formulation, gastric residual at 2 hours was 15.9 ± 6.2 % (SEM) and colonic filling 49.6 ± 7.8 % of administered dose; in 11/12 participants, liquid had entered colon within 2 hours.
The time of arrival of radiolabeled capsule in the colon was 5.16 ± 0.46 hours (SEM), with a range of 2–8 hours and a mode at 6 hours.
Urine Excretion Profile after Oral Liquid or Capsule Administration of Sugars
Figure 3 shows a summary of mass of mannitol excreted per hour after capsule and liquid formulation; note that after capsule formulation, the mannitol excretion lags significantly behind excretion after liquid formula, consistent with the later release of the sugars to the absorbing epithelium with the capsule formulation. Though we administered 5 times as much lactulose as mannitol, there was a higher amount of mannitol than lactulose excreted with the two forms of administration. There is marked overlap in the percentage of lactulose excreted (in each urine aliquot) after liquid formulation, and there is virtually no excretion of the sugars during the first 2 hours after capsule administration. Sugars start to appear in the urine in appreciable amounts about 4 hours after capsule administration and this corresponds with the average time of arrival of capsule content in the colon of ~5 hours.
Figure 3.
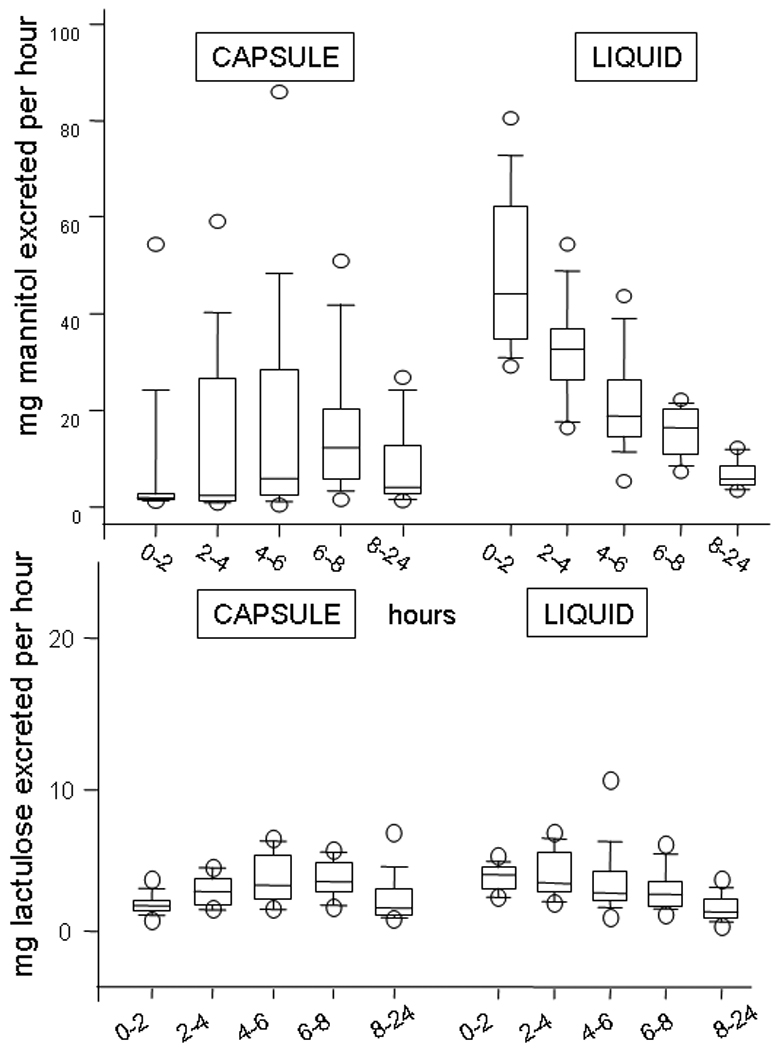
Summary of mass of mannitol and lactulose excreted per hour after capsule and liquid formulation; note that, after capsule formulation, the mannitol excretion lags significantly behind excretion after liquid formula, consistent with the delivery of the sugars to the absorbing small intestine or colon. While the data from liquid formulation suggest greater absorption earlier, this may simply reflect the relative amount of mannitol available for absorption from the liquid formulation compared to the capsule.
The percentages of mannitol excreted per hour were 0.405% (95% CI 0.27, 1.28) with capsule at 8–24 hours, 0.375% (0.26, 0.67) at 8–24 hours with liquid formulation, and 4.35% (95% CI 3.365, 6.220) with liquid formulation after 0–2 hours.
In Figure 4, group data show the cumulative percentage of mannitol and lactulose excreted after administration in capsule or in liquid formulation (n=12 each). For each time period (0–2, 2–4, 4–6, and 6–8 hours) a significant sugar by vehicle interaction effect was detected (all p<0.001), indicating a differential greater proportionate excretion via liquid formulation than capsule delivery for mannitol compared to lactulose. For the period 8–24 hours, no interaction was detected (p=0.32) and there was no effect of mode of delivery (liquid vs. capsule, p=0.31), but a difference in the proportionate excretion of lactulose vs. mannitol was observed (p<0.001). A modest order effect (p=0.03) was observed for the excretion of mannitol, which was roughly consistent across the time periods and, overall, resulted in a difference of approximately 5% greater excretion when liquid was used first.
Figure 4.
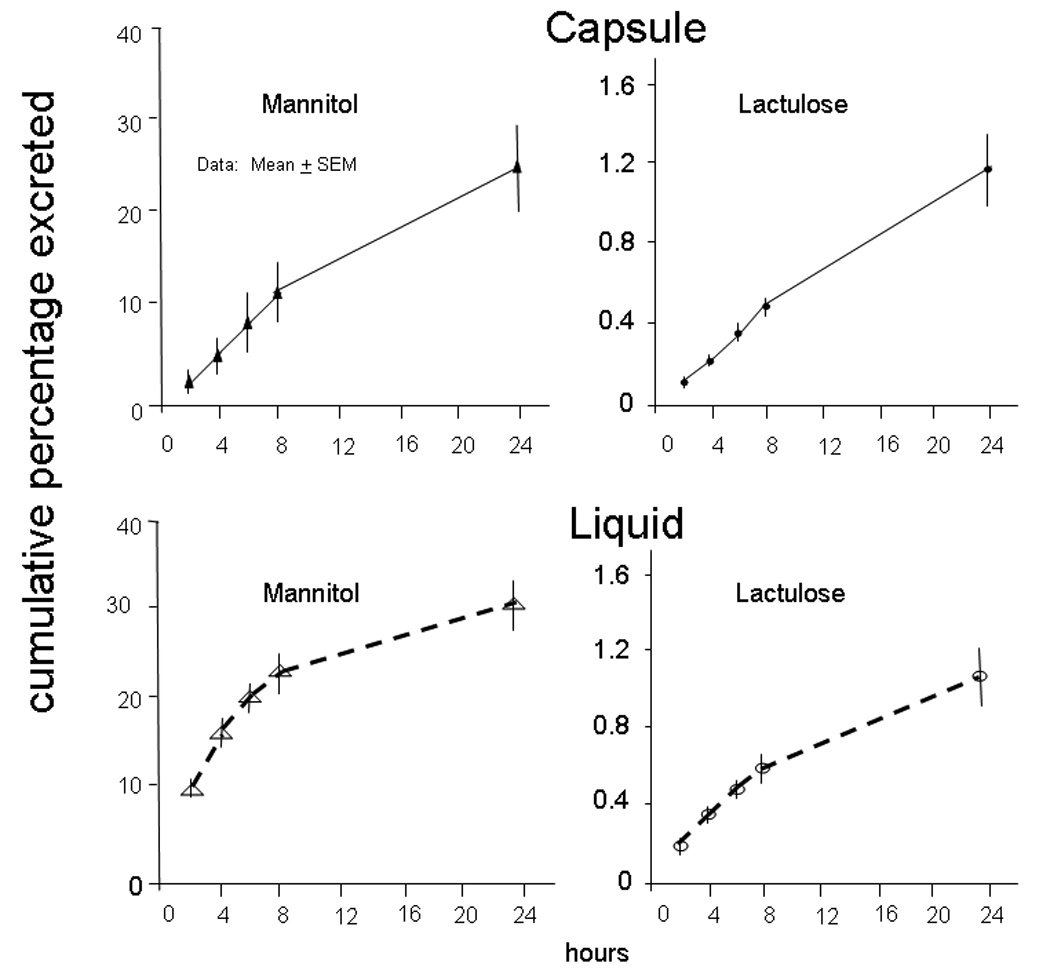
Proportionate excretion of lactulose and mannitol after administration via capsule (upper panel) or liquid formulation (lower panel). The mass of mannitol absorbed and excreted is greater for mannitol even though there was 5 times as much lactulose ingested. The cumulative average excretion of mannitol reaches less than 300 mg, suggesting that at least 700 mg of mannitol is available for absorption between 8 and 24 hours after liquid formulation.
The average cumulative excretion of mannitol reaches less than 300 mg at 6 hours, suggesting that at least 700 mg of mannitol are available for absorption between 6 and 24 hours after liquid formulation or capsule. For each type of sugar separately, no significant time by type of delivery interaction was detected. However, the mode of delivery significantly influenced the amount of mannitol excreted, with the liquid formulation resulting in greater proportionate excretion (p=0.01) compared to capsule (average over all time points 19% with liquid, and 9% with capsule formulation). The overall effect of mode of delivery on cumulative excretion of lactulose was not significant (average over all time points 0.5% with liquid, and 0.4% with capsule formulation, p=0.53).
Urine Lactulose: Mannitol Ratio
Figure 5 shows urine LMR excretion every 2 hours from 0–8 hours and from 8–24 hours. for the administration of sugars in aqueous solution. Data for capsule formulation are from 6–8 hours and 8–24 hours, since the capsule had not reached colon for >5 hours (average).
Figure 5.
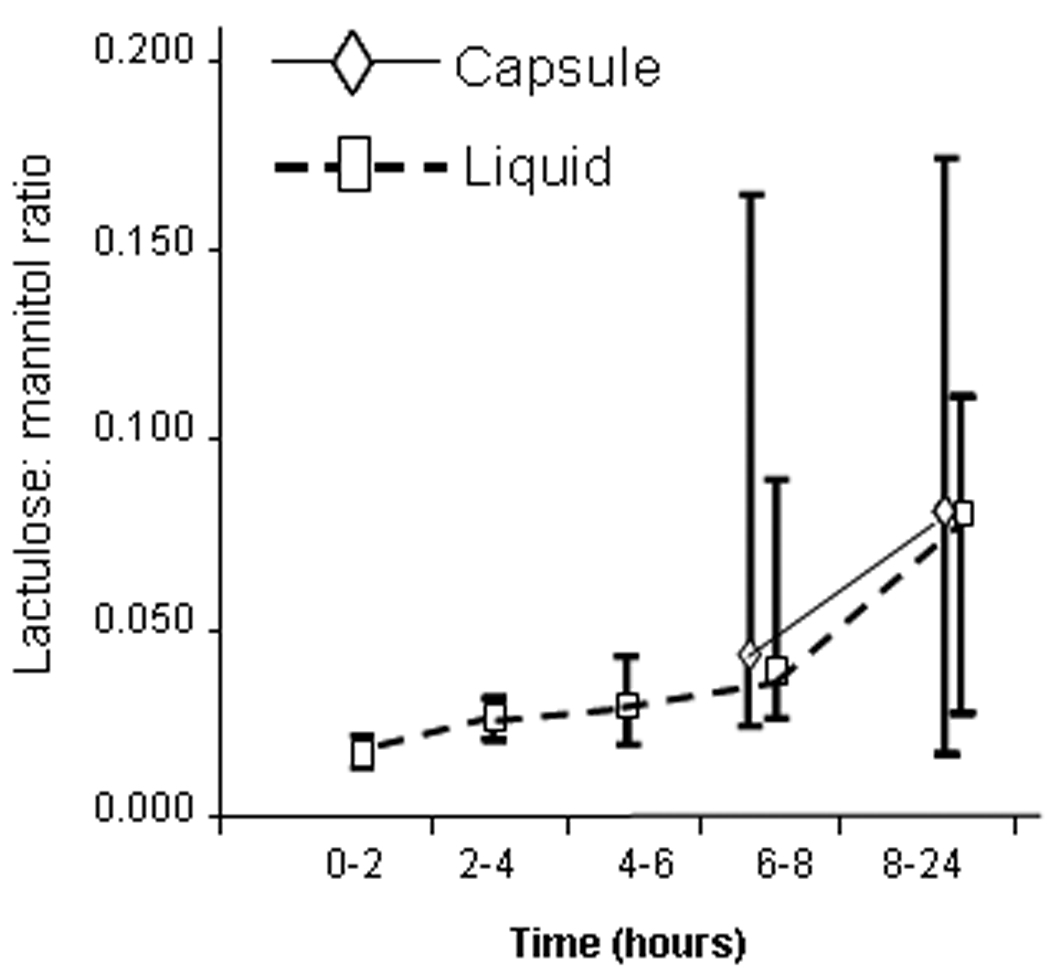
Urine excretion of mannitol and lactulose in aliquots from the total urine output obtained every 2 hours from 0–8 hours and from 8–24 hours. All data are provided for the administration of sugars in aqueous solution. Amounts of sugars excreted after capsule formulation are summarized from 6–8 hours and from 8–24 hours.
The repeated measures AOV of the (log) ratios indicated a significant time by type of delivery interaction (p=0.003). In particular, with the liquid formulation, the 0–2 hour LMR differed from the LMR at 2–4, 4–6, 6–8, and 8–24 hours (all p<0.01). No time effects were observed for capsule delivery (p=0.44). This is consistent with the greater absorption from the liquid formulation in the first 2 hours than subsequently, when imaging studies showed that sugars were in the colon. Furthermore, the calculated LMRs via capsule delivery at 6–8 hours and 8–24 hours (consistent with location of the sugars in the colon) differed (p<0.005) from the ratio at 0–2 hours after liquid formulation (reflecting small intestine and, possibly, colonic permeability since average 45% of the oral liquid reached the colon by 2 hours). The LMRs at 8–24 hours via either method of delivery were not significantly different (Figure 5 and Figure 6).
Figure 6.
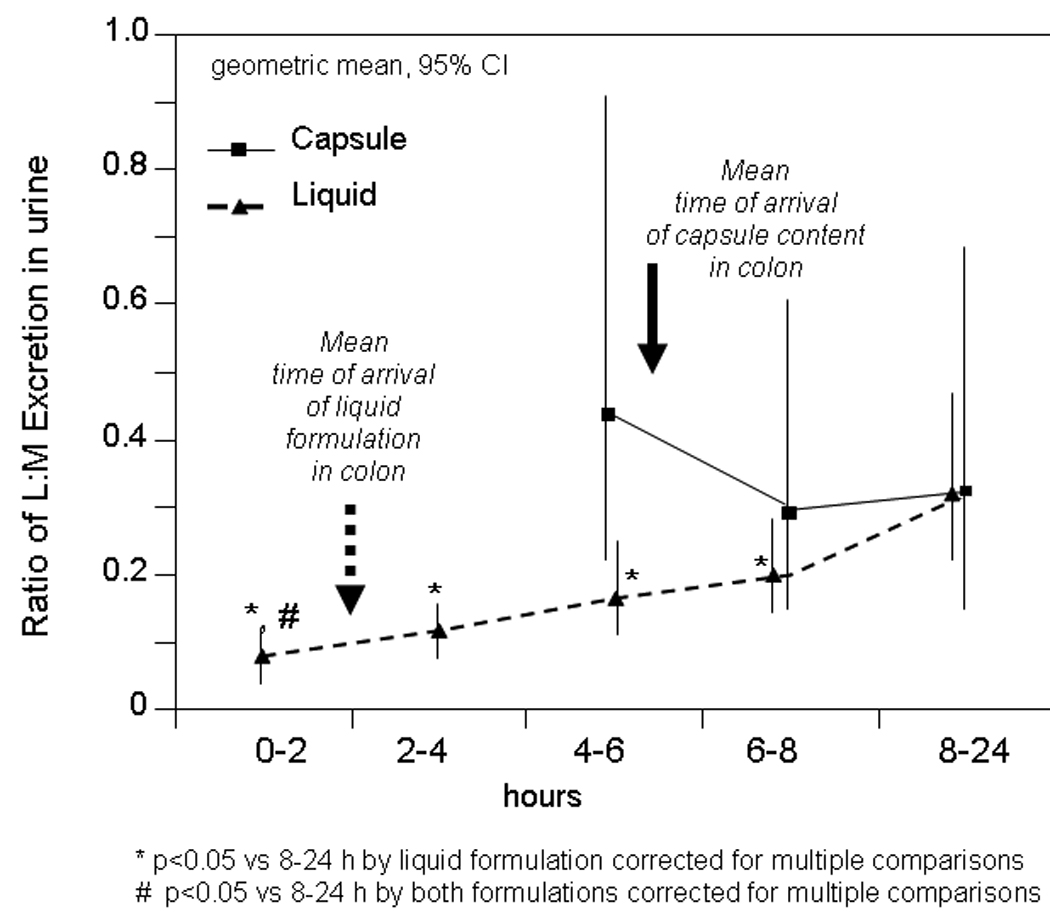
Comparison of the lactulose to mannitol ratio at different times using the two formulations. Data show the geometric means and 95% confidence intervals. After liquid formulation, the ratio is higher at 8–24 hours than with all other urine samples. Note also that the ratio is higher for the urine samples after liquid and capsule formulations at 8–24 hours compared with 0–2 hours with the liquid formulation. These data suggest colonic (6–8 hours or 8–24 hours) permeability is higher than small bowel permeability (0–2 hours). There were no significant differences in ratios at 6–8 hours and 8–24 hours with either formulation, and there were no differences between the different formulations at these times.
The L: M ratio in the urine collected between 8 and 24 hours after administration in the capsule reflects colonic permeability, since the isotope from the two formulations was located entirely in the colon after 8 hours. The significantly lower urine LMR during the 0–2 hour urine collection, compared to the 8–24 hour collections for both liquid formulation and capsule delivery [all p<0.05 (adjusted) compared to 0–2 hour urine] would initially suggest that colonic permeability is greater than the predominantly small intestinal permeability measured at 0–2 hours.
DISCUSSION
We have applied LC-MS/MS analysis to assess intestinal permeability by the differential urine excretion of sugars. LC-MS/MS analysis shows validity compared with an enzymatic method. Analytical performance of the LC-MS/MS method, which included intra- and inter-assay precision, linearity, and recovery by proportional mixing, was validated and subsequently used to compare the LMR between liquid or delayed-release capsule formulations.
Our data show that the excretion rate is consistent with prior data in the literature, with higher urinary excretion of the monosaccharide mannitol than the disaccharide lactulose at all times of observation. This is consistent with the concept that small intestinal permeability is greater than colonic permeability. However, there is a significant impact of the time of urine collection on the interpretation of the single sugar excretion as well as the urinary LMR. Simultaneous abdominal imaging of radioisotopes incorporated with the different formulations shows that, during the first few hours after ingestion, there is inter-individual variation in the location of the sugars in the gastrointestinal tract. In the past, the 0–6 hour collection was assumed to reflect small bowel permeability, or 0–3 hour for upper and 3–5 hour for lower small bowel permeability. Since an average 49.6% of isotopically labeled liquid was located in the colon at 2 hours, it follows that, during the first 2 hours, sugars may be absorbed in part from the colon rather than exclusively in the small intestine. It is conceivable that ingestion of the sugars with a meal might slow the transit of the sugars through the small intestine; however, in the absence of simultaneous imaging, the location of the sugars over time could not be predicted or assumed to be standard between individuals or even in the same subject studied on separate occasions. Understanding the location of liquid formulation during the standard 2-sugar permeability test suggests the need for caution in the interpretation of the excretion profiles. In fact, in order to collect urine samples that reflect small intestinal permeability, it may prove necessary to obtain urine samples every 30 minutes over the first 2 hours, or to conduct simultaneous imaging in order to identify the urine samples that correspond to the time when none of the isotope (and sugars) reach the colon.
We observed that the later urine collections had higher LMRs, and this may initially suggest that the permeability of the colon is greater than that of the small intestine. This inference is contrary to the observations with mannitol and the published literature. We considered two potential explanations for the higher LMRs in the later urine samples.
First, it is conceivable that so much of the mannitol was absorbed by the small intestine that there was a lower likelihood to absorb mannitol in the colon, in contrast to the availability of lactulose for absorption in the colon. This is unlikely to be the only explanation, since we estimated (from the cumulative excretion plots in Figure 4) that there was >70% of the mannitol delivered to the colon and we observed very similar results in LMRs (see Figure 6) and amount of mannitol excreted per hour (see Figure 3) between 8 and 24 hours with the liquid and capsule formulations.
Second, we considered that the lactulose and mannitol may be metabolized to a different extent (e.g. mannitol > lactulose) by colonic bacteria, with greater availability of lactulose and a higher urine LMR in the 8–24 hour collections compared to the 0–2 hour urine. Ideally, probes used to measure colonic permeability should be stable in the colonic environment. Mannitol and lactulose are fermented by colonic bacteria; studies have explored the fraction of different sugars remaining after in vitro degradation by rat colonic contents. Thus, Meddings and Gibbons showed that 25.9 ± 23% (SEM) of the mannitol and 25.9 ± 10% of the lactulose remained after incubation in an environment that simulated rat colon (20). However, the LC/MS-MS method specifically measures the sugars of interest with high sensitivity rather than their bacterial metabolites. Our experiments showed recovery of both intact sugars in the urine during the 8–24 hour period, confirming that the intact sugars were available for absorption in the human colon. We plan to conduct further studies of the potential role of differential bacterial metabolism on the absorption of these sugars by comparing the excretion of each individual sugar and the differential excretion of the two sugars after oral ingestion with the colon unprepared, and after injection of the sugars into the right colon after thorough colonic cleansing. These studies will also incorporate sucralose, which was not metabolized in an environment that simulated rat colon (20).
There are alternative approaches for permeability measurement.
We have worked on differential excretion of sugars due to several advantages: noninvasive, in vivo, scalability, batch processing, and estimation of differential excretion to avoid the influence of interindividual variations that may affect single probe permeability measurements, as with 51CrEDTA.
Studies with 51Cr-EDTA had proposed different times of urine collection to try to map permeability of distinct parts of the gut (5,10,21). In one study (21), 51Cr-EDTA in a 24-hour urine collection was proposed as an ideal marker for increased colonic permeability in ulcerative colitis. This radioisotope is not approved for use in the United States.
Studies of permeability function in colonic mucosal biopsies using Ussing chambers (22–25) do not provide in vivo assessment of permeability. Such mucosal biopsies lose intrinsic neural input (e.g., from submucosal neurons), and there may be local inflammation which may be chemical (e.g., cytokines) rather than cellular and, therefore, may not be detected histologically. Muscarinic cholinergic activation, glial function, and local inflammation influence mucosal permeability (26,27). Therefore, a robust in vivo method to selectively assess colonic mucosal permeability is desirable to facilitate further research of mucosal function.
Overall, our data confirm the principle that small bowel permeability is greater than colonic permeability, based on the urine collections that correspond to the time when the sugars were located predominantly in the organs of interest. Thus, the proportions of mannitol excreted per hour were 0.405 (95% CI 0.27, 1.28) with capsule at 8–24 hours, 0.375 (0.26, 0.67) at 8–24 hours with liquid formula, and 4.35 (95% CI 3.365, 6.220) with liquid formulation after 0–2 hours.
Since the excretion rate of lactulose does not increase over time, the data are consistent with low permeability of intestinal and colonic mucosa to lactulose. On the other hand, the LMR is higher for the 8–24 hour urine collections by both formulations compared to the LMR during 0–2 hour after liquid formulation. This would suggest that colonic mucosal permeability is higher than small intestinal permeability, an interpretation that is not supported by the excretion profile of the smaller molecular weight sugar, mannitol compared to the larger lactulose in our study. Thus, Figure 3 shows there was still greater excretion of mannitol than lactulose during the 8–24 hour period after liquid formulation (mannitol 0.375%/hour [IQR 0.26–0.67] and lactulose 0.025%/hour [0.020–0.040]), despite the fact that there was a 5-fold greater mass of lactulose ingested. We suggest that the higher LMR at 8–24 hours compared to 0–2 hours simply reflects the relatively lower absorption of mannitol in the colon than in the small intestine, whereas the lactulose excretion is relatively constant over time. In view of the potential pitfall in its interpretation, we question whether LMR at 8–24 hours should be used to measure colonic mucosal permeability.
In summary, in vivo intestinal permeability measurements using excretion of lactulose and mannitol indicate that the urine profile at 0–2 hours reflects, in part, colonic and not exclusively small bowel permeability. The 0 to 5 or 6 hour differential 2-sugar urine excretion ratio that is used extensively to measure small intestinal permeability should be interpreted with caution. The delayed-release capsule delivery allows more mannitol to reach the colon to avoid absorption of mannitol from the small intestine. It is unclear whether this would allow for more accurate measurements of colonic mucosal permeability. This requires further validation including comparison of urine sugar excretion and LMR with capsule, liquid formulation and local delivery of the sugars to the right colon. When LMRs suggest greater colonic than small bowel permeability, they are contrary to observations with the smaller sugar molecule, mannitol. We believe that LMRs need to be interpreted with caution.
Acknowledgements
Dr. Camilleri is supported in part by grants RO1-DK54681 and K24-DK02638 from National Institutes of Health. The study was enabled by the GI Imaging and Physiology Core and the Nursing Core of the Mayo Clinic CTSA grant (NIH RR0024150). We thank Professor Sidney F. Phillips for helpful discussions and Mrs. Cindy Stanislav for excellent secretarial assistance.
APPENDIX
A. Measurement of Urine Sugars
Lactulose, mannitol, and uniformly labeled 13C6-Glucose were obtained from Sigma-Aldrich (St. Louis, MO 63103). From 10 mg/mL stock solutions of lactulose and mannitol, 10 point calibration curves for each analyte were made from 0 to 1000 µg/mL in 2:98 acetonitrile-water. A 20 mg/mL 13C6-Glucose working internal standard was made in 2:98 acetonitrile-water.
Fifty µL of 13C6-Glucose internal standard was added to 50 µL of urine sample or standard. The samples were diluted 1/40 by adding 2 mL of water. To remove the sample’s matrix effects, 4 mL of dichloromethane were added and then vortex-mixed for 1 minute. The samples were allowed to incubate for 30 minutes at room temperature and then vortex-mixed for 1 minute before being placed in a centrifuge for 10 minutes at 3,500 rpm. The final dilution of 1/400 was made by transferring 100 µL of supernatant to a test tube containing 1 mL of 85:15 acetonitrile-water and then vortex-mixed for 20 seconds.
Urine sugar concentrations were measured by liquid chromatography-tandem mass spectrometry using an adapted approach to that recently reported in the literature (14) and expressed as the average of two separate runs. The high performance liquid chromatography tandem mass spectrometry (HPLC-MS/MS) system included an API 5000 triple-quadruple mass spectrometer (Applied Biosystems/MDS SCIEX, Foster City, CA, USA/Concord, Ontario, Canada) coupled with electrospray ionization (ESI) source that was operated at 550° C in the negative ion mode. Sugars were separated chromatographically using a 250 × 4.6 mm Phenomenex Luna 5µm NH2 column (Phenomenex, Torrance, California) with a tailored normal phase liquid chromatography (LC) method as shown in Appendix Table I. The LC organic mobile phase consisted of acetonitrile and the aqueous mobile phase water; both were spiked with 0.1% formic acid and sonicated to degas. The injection volume was 50 µL, and the total analysis time was 5 minutes. The Q1 and Q3 quadruples, collision energy (CE), declustering potential (DP), entrance potential (EP), and collision cell exit potential (CXP) were all tuned for high sensitivity mass resolution as summarized in Appendix Table II. Sugar peaks were identified and measured using Analyst 1.4.1 software package (MDS SCIEX, Concord, Ontario, Canada).
Appendix Table I.
Monitored Ion Transitions and their Parameter Settings
| Analyte (Q1) (m/z, amu) |
Fragment (Q3) (m/z, amu) |
DP (volt) |
EP (volt) |
CE (volt) |
CXP (volt) |
|---|---|---|---|---|---|
| Mannitol (181.05) | 89.00 | −100.00 | −10.00 | −21.00 | −10.00 |
| Mannitol (181.05) | 101.00 | −100.00 | −10.00 | −21.50 | −12.00 |
| Mannitol (181.05) | 119.05 | −100.00 | −10.00 | −17.50 | −14.50 |
| Mannitol (181.05) | 163.10 | −100.00 | −10.00 | −17.00 | −10.00 |
| Lactulose (341.10) | 101.00 | −105.00 | −10.00 | −24.00 | −11.50 |
| Lactulose (341.10) | 161.05 | −105.00 | −10.00 | −12.50 | −10.00 |
| Lactulose (341.10) | 179.15 | −105.00 | −10.00 | −12.00 | −11.50 |
| 13C6-Glucose (185.20) | 92.00 | −150.00 | −10.00 | −21.00 | −15.00 |
| 13C6-Glucose (185.20) | 105.00 | −150.00 | −10.00 | −21.00 | −15.00 |
| 13C6-Glucose (185.20) | 123.00 | −150.00 | −10.00 | −21.00 | −15.00 |
Appendix Table II.
Liquid Chromatography Method Summary
| Column: | Phenomenex Luna 5u NH2 100A 250×4.60 mm | |||
| Elute Solvent A: | Acetonitrile with 0.1% formic acid | |||
| Elute Solvent B: | H2O with 0.1% formic acid | |||
| Data Window Start: | 15 sec | |||
| Data Window Length | 300 sec | |||
| Step: | 1 | 2 | 3 | 4 |
| Time (sec): | 30 | 120 | 60 | 120 |
| Flow Rate (mL/min): | 1.8 | 1.2 | 1.2 | 1.8 |
| Flow Type: | Step | Ramp | Ramp | Ramp |
| % Elute A | 80.0 | 30.0 | 70.0 | 80.0 |
| % Elute B | 20.0 | 70.0 | 30.0 | 20.0 |
B. Statistical Power
Previous data in the literature (8) indicate the between subjects variation in excretion ratios is 0.0021 with a mean of 0.0088 (CV = 24%). Since at the start of these studies we did not have an estimate of the variation in (within subject) deltas (Capsule vs. Liquid) of L:M ratios, we assumed that the variation in deltas was at most the same as the between subject variation in L:M ratios. Thus, using a subject as their own control is often assumed to provide smaller variation in treatment differences, but we developed our study sample size assuming the variation would be greater and analogous to that between rather than within subjects. This precaution was taken to ensure we did not under-power our proposed crossover study. Indeed, using an SD=0.0021 (8), there was approximately 91% power to detect a difference of 0.0022 (vs. a zero difference in L:M ratios) between liquid and capsule delivery with n=12 using a paired t-test. The difference detectable is 25% of the literature (8) reported mean of L:M ratio for direct delivery (liquid formulation). Thus, the study was powered to demonstrate that the mean 0–6 hour excretion ratios of the two sugars differed by 25% in a crossover study using a paired analysis.
RESULTS
Performance of the LC-MS/MS Method
Linearity and detection limits
Linear responses were observed in the concentration range of 1–1000 µg/mL for mannitol and 1–400 µg/mL for lactulose. Samples showed good linearity with dilution. The observed (experimental) linearity values plotted against the expected values gave slopes and correlation coefficients that approximated 1.0 for lactulose (slope 0.976 and r2 0.9994) and mannitol (slope 1.018 and r2 0.9953). The detection limit of the assay was 1.7 µg/mL for lactulose and 0.035 µg/mL for mannitol.
Precision and recovery
The intra- and inter-assay precision data (summarized in Appendix Table III) were assessed for the LC-MS/MS method at concentrations of 30–200 µg/mL for lactulose and 40–200 µg/mL for mannitol by analyzing (on 8 separate occasions) three different lactulose and mannitol contents in urine (low, medium, high). The assays were conducted on 5 separate days. Intra-assay coefficients of variation (CVs) averaged 11.3% for lactulose (range, 10.1 – 13.6%) and 17.7% for mannitol (range, 14.2 – 21.9%). Inter-assay CVs averaged 22% (range, 17.7 – 24.9%) for lactulose and 16.2% for mannitol (range, 13.6 – 18.9%).
Appendix Table III.
Precision of the Assay in Urine: Intra- and Inter-assay Precision Analyzing 3 Concentration Levels of Lactulose and Mannitol in Urine (CV = coefficient of variation)
| Within-run | Lactulose | Mannitol | ||
|---|---|---|---|---|
| Mean ± SD (ug/mL) | CV % | Mean ± SD (ug/mL) | CV % | |
| Low | 36.0 ± 3.7 | 10.2 | 47.7 ± 10.4 | 21.9 |
| Medium | 100.1 ± 10.1 | 10.1 | 99.6 ± 16.9 | 17.0 |
| High | 175.6 ± 23.9 | 13.6 | 160.1 ± 22.7 | 14.2 |
| Between-run | ||||
| Mean ± SD (ug/mL) | CV % | Mean ± SD (ug/mL) | CV % | |
| Low | 30.1 ± 5.3 | 17.7 | 48.6 ± 9.2 | 18.9 |
| Medium | 93.7 ± 23.4 | 24.9 | 98.1 ± 15.9 | 16.2 |
| High | 158.3 ± 37.2 | 23.5 | 161.0 ± 21.8 | 13.6 |
Recovery was determined using a blending technique of three urine samples with known concentrations of lactulose and mannitol. Prior to diluting or extracting, two of the urine samples were combined in different proportions (75/25, 50/50, 25/75). The recovery was determined by measuring the sugar concentration in the samples after the combination, then dividing by the expected concentration. The average recovery of lactulose was 106% and mannitol 104% (Appendix Table IV). Recovery estimates by spiking the purified analytes into the urine matrix were not as good as the proportional mixing, although recovery at the low concentration (25 µg/ml) was within acceptable limits: 87% for lactulose and 90% for mannitol. Spike recoveries at higher concentrations (50 or 100 µg/ml) were below 80% for both sugars.
Appendix Table IV.
Analytical Recovery of Lactulose and Mannitol in Urine
| Lactulose | Mannitol | ||||
|---|---|---|---|---|---|
| Proportion Added |
Found, mean ± SD (µg/mL) |
Recovery % |
Proportion Added |
Found, mean ± SD (µg/mL) |
Recovery % |
| Low | 31.8 ± 3.7 | Low | 40.2 ± 4.2 | ||
| 75/25 | 39.2 ± 10.5 | 93 | 75/25 | 52.1 ± 16.4 | 101 |
| 50/50 | 59.1 ± 1.7 | 113 | 50/50 | 58.8 ± 1.7 | 93 |
| 25/75 | 76.6 ± 5.2 | 122 | 25/75 | 67.3 ± 3.1 | 90 |
| Med | 72.8 ± 10.1 | Med | 86.0 ± 4.5 | ||
| Low | 31.8 ± 3.7 | Low | 40.2 ± 4.2 | ||
| 75/25 | 54.7 ± 6.5 | 93 | 75/25 | 70.3 ± 3.7 | 108 |
| 50/50 | 83.9 ± 21.9 | 98 | 50/50 | 93.7 ± 1.6 | 104 |
| 25/75 | 118.5 ± 10.6 | 105 | 25/75 | 104.2 ± 13.9 | 90 |
| High | 139.8 ± 23.9 | High | 140.8 ± 11.2 | ||
| Med | 72.8 ± 10.1 | Med | 86.0 ± 4.5 | ||
| 75/25 | 101.5 ± 0.7 | 113 | 75/25 | 93.8 ± 8.1 | 144 |
| 50/50 | 118.0 ± 8.5 | 111 | 50/50 | 93.3 ± 3.3 | 103 |
| 25/75 | 128.0 ± 15.6 | 104 | 25/75 | 119.5 ± 13.4 | 103 |
| High | 139.8 ± 23.9 | High | 140.8 ± 11.2 | ||
| MEAN | 106 | 104 | |||
Accuracy of LC-MS Method Relative to Commercial Assay
In order to assess the accuracy and validity of the measurements by HPLC-MS/MS, 80% of the samples were sent to a commercial laboratory (Genova Diagnostics, Asheville, NC). Comparisons of sugar excretion showed that the LC-MS/MS method trended with the commercial enzyme assay-based method for both analytes and for both administration methods (Figure 1). The LC-MS/MS method did not show saturation at later time points in the urine collections; this saturation was observed in several samples at the later urine collections with the commercial assay (data not shown). Accuracy is assessed using the Bland-Altman analysis using the information from studies conducted with either the liquid or capsule formulations. For the 0–2 hour collections, we included only the observations obtained with the liquid formulation. For the 8–24 hour collections, the data in Figure 2 provide all information obtained by liquid or capsule formulations. As expected, the variation is somewhat higher relative to median concentration for lactulose, than for mannitol, since the excretion of lactulose is about 10% that of mannitol. The differences between the two methods were within two standard deviations except for a few points (Figure 2).
Contributor Information
Michael Camilleri, Clinical Enteric Neuroscience Translational and Epidemiological Research (CENTER) Group, College of Medicine, Mayo Clinic, Rochester, MN
Ashley Nadeau, Clinical Enteric Neuroscience Translational and Epidemiological Research (CENTER) Group, College of Medicine, Mayo Clinic, Rochester, MN
Jesse Lamsam, Immunochemistry Core Laboratory, College of Medicine, Mayo Clinic, Rochester, MN.
Sara Linker Nord, Clinical Enteric Neuroscience Translational and Epidemiological Research (CENTER) Group, College of Medicine, Mayo Clinic, Rochester, MN.
Michael Ryks, Clinical Enteric Neuroscience Translational and Epidemiological Research (CENTER) Group, College of Medicine, Mayo Clinic, Rochester, MN.
Duane Burton, Clinical Enteric Neuroscience Translational and Epidemiological Research (CENTER) Group, College of Medicine, Mayo Clinic, Rochester, MN.
Seth Sweetser, Clinical Enteric Neuroscience Translational and Epidemiological Research (CENTER) Group, College of Medicine, Mayo Clinic, Rochester, MN
Alan R. Zinsmeister, Division of Biomedical Statistics and Informatics, College of Medicine, Mayo Clinic, Rochester, MN.
Ravinder Singh, Immunochemistry Core Laboratory, College of Medicine, Mayo Clinic, Rochester, MN
REFERENCES
- 1.Porras M, Martin MT, Yang PC, Jury J, Perdue MH, Vergara P. Correlation between cyclical epithelial barrier dysfunction and bacterial translocation in the relapses of intestinal inflammation. Inflamm Bowel Dis. 2006;12:843–852. doi: 10.1097/01.mib.0000231571.88806.62. [DOI] [PubMed] [Google Scholar]
- 2.Sun Z, Wang X, Andersson R. Role of intestinal permeability in monitoring mucosal barrier function. History, methodology, and significance of pathophysiology. Dig Surg. 1998;15:386–397. doi: 10.1159/000018651. [DOI] [PubMed] [Google Scholar]
- 3.Bjarnason I, MacPherson A, Hollander D. Intestinal permeability: an overview. Gastroenterology. 1995;108:1566–1581. doi: 10.1016/0016-5085(95)90708-4. [DOI] [PubMed] [Google Scholar]
- 4.Camilleri M, Gorman H. Intestinal permeability and irritable bowel syndrome. Neurogastroenterol Motil. 2007;19:545–552. doi: 10.1111/j.1365-2982.2007.00925.x. [DOI] [PubMed] [Google Scholar]
- 5.Dunlop SP, Hebden J, Campbell E, Naesdal J, Olbe L, Perkins AC, Spiller RC. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am J Gastroenterol. 2006;101:1288–1294. doi: 10.1111/j.1572-0241.2006.00672.x. [DOI] [PubMed] [Google Scholar]
- 6.Marshall JK, Thabane M, Garg AX, Clark W, Meddings J, Collins SM. Intestinal permeability in patients with irritable bowel syndrome after a waterborne outbreak of acute gastroenteritis in Walkerton, Ontario. Aliment Pharmacol Ther. 2004;20:1317–1322. doi: 10.1111/j.1365-2036.2004.02284.x. [DOI] [PubMed] [Google Scholar]
- 7.Tibble JA, Sigthorsson G, Foster R, Forgacs I, Bjarnason I. Use of surrogate markers of inflammation and Rome criteria to distinguish organic from nonorganic intestinal disease. Gastroenterology. 2002;123:450–460. doi: 10.1053/gast.2002.34755. [DOI] [PubMed] [Google Scholar]
- 8.Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M, Neal KR. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804–811. doi: 10.1136/gut.47.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bjarnason I, O’Morain C, Levi AJ, et al. Absorption of 51chromium-labeled ethylenediaminetetraacetate in inflammatory bowel disease. Gastroenterology. 1983;85:318–322. [PubMed] [Google Scholar]
- 10.Bjarnason I, Peters TJ, Veall N. A persistent defect in intestinal permeability in celiac disease demonstrated by a 51Cr-labelled EDTA absorption test. Lancet. 1983;1:323–325. doi: 10.1016/s0140-6736(83)91628-8. [DOI] [PubMed] [Google Scholar]
- 11.Maxton DG, Bjarnason I, Reynolds AP, et al. Lactulose, 51Cr-labelled ethylenediaminetetra-acetate, L-rhamnose and polyethyleneglycol 400 [corrected] as probe markers for assessment in vivo of human intestinal permeability. Clin Sci (Lond) 1986;71:71–80. doi: 10.1042/cs0710071. [DOI] [PubMed] [Google Scholar]
- 12.Camilleri M, Brown ML, Malagelada JR. Impaired transit of chyme in chronic intestinal pseudoobstruction. Correction by cisapride. Gastroenterology. 1986;91:619–626. doi: 10.1016/0016-5085(86)90631-1. [DOI] [PubMed] [Google Scholar]
- 13.Bouras EP, Burton DD, Camilleri M, Stephens DA, Thomforde GM. Effect of cyclooxygenase-2 inhibitors on gastric emptying and small intestinal transit in humans. Neurogastroenterol Motil. 2004;16:729–735. doi: 10.1111/j.1365-2982.2004.00547.x. [DOI] [PubMed] [Google Scholar]
- 14.Proano M, Camilleri M, Phillips SF, Brown ML, Thomforde GM. Transit of solids through the human colon: regional quantification in the unprepared bowel. Am J Physiol. 1990;258:G856–G862. doi: 10.1152/ajpgi.1990.258.6.G856. [DOI] [PubMed] [Google Scholar]
- 15.Lostia AM, Lionetto L, Principessa L, Evangelisti M, Gamba A, Villa MP, Simmaco M. A liquid chromatography/mass spectrometry method for the evaluation of intestinal permeability. Clin Biochem. 2008;41:887–892. doi: 10.1016/j.clinbiochem.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 16.Behrens RH, Docherty H, Elia M, Neale G. A simple enzymatic method for the assay of urinary lactulose. Clinica Chimica Acta. 1984;137:361–367. doi: 10.1016/0009-8981(84)90125-6. [DOI] [PubMed] [Google Scholar]
- 17.Lunn PG, Northrop CA, Northrop AJ. Automated enzymatic assays for the determination of intestinal permeability probes in urine. 2. Mannitol. Clinica Chimica Acta. 1989;183:163–170. doi: 10.1016/0009-8981(89)90332-x. [DOI] [PubMed] [Google Scholar]
- 18.Lunn PG, Northrop-Clewes CA. Intestinal permeability: update on the enzymatic assay of mannitol. Letter to the Editor. Clinica Chimica Acta. 1992;205:151–152. doi: 10.1016/s0009-8981(05)80011-7. [DOI] [PubMed] [Google Scholar]
- 19.Northrop CA, Lunn PG, Behrens RH. Automated enzymatic assays for the determination of intestinal permeability probes in urine. 1. Lactulose and lactose. Clinica Chimica Acta. 1990;187:79–88. doi: 10.1016/0009-8981(90)90333-n. [DOI] [PubMed] [Google Scholar]
- 20.Meddings JB, Gibbons I. Discrimination of site-specific alterations in gastrointestinal permeability in the rat. Gastroenterology. 1998;114:83–92. doi: 10.1016/s0016-5085(98)70636-5. [DOI] [PubMed] [Google Scholar]
- 21.Jenkins AP, Nukajam WS, Menzies IS, Creamer B. Simultaneous administration of lactulose and 51Cr-ethylenediaminetetraacetic acid. A test to distinguish colonic from small-intestinal permeability change. Scand J Gastroenterol. 1992;27:769–773. doi: 10.3109/00365529209011181. [DOI] [PubMed] [Google Scholar]
- 22.Münch A, Ström M, Söderholm JD. Dihydroxy bile acids increase mucosal permeability and bacterial uptake in human colon biopsies. Scand J Gastroenterol. 2007;42:1167–1174. doi: 10.1080/00365520701320463. [DOI] [PubMed] [Google Scholar]
- 23.Piche T, Barbara G, Aubert P, Bruley des Varannes S, Dainese R, Nano JL, Cremon C, Stanghellini V, De Giorgio R, Galmiche JP, Neunlist M. Impaired intestinal barrier integrity in the colon of irritable bowel syndrome patients: involvement of soluble mediators. Gut. 2008 Sep 29; doi: 10.1136/gut.2007.140806. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 24.Wallon C, Braaf Y, Wolving M, Olaison G, Söderholm JD. Endoscopic biopsies in Ussing chambers evaluated for studies of macromolecular permeability in the human colon. Scand J Gastroenterol. 2005;40:586–595. doi: 10.1080/00365520510012235. [DOI] [PubMed] [Google Scholar]
- 25.Wallon C, Yang PC, Keita AV, Ericson AC, McKay DM, Sherman PM, Perdue MH, Söderholm JD. Corticotropin-releasing hormone (CRH) regulates macromolecular permeability via mast cells in normal human colonic biopsies in vitro. Gut. 2008;57:50–58. doi: 10.1136/gut.2006.117549. [DOI] [PubMed] [Google Scholar]
- 26.Cameron HL, Perdue MH. Muscarinic acetylcholine receptor activation increases transcellular transport of macromolecules across mouse and human intestinal epithelium in vitro. Neurogastroenterol Motil. 2007;19:47–56. doi: 10.1111/j.1365-2982.2006.00845.x. [DOI] [PubMed] [Google Scholar]
- 27.Savidge TC, Newman P, Pothoulakis C, Ruhl A, Neunlist M, Bourreille A, Hurst R, Sofroniew MV. Enteric glia regulate intestinal barrier function and inflammation via release of S-nitrosoglutathione. Gastroenterology. 2007;132:1344–1358. doi: 10.1053/j.gastro.2007.01.051. [DOI] [PubMed] [Google Scholar]


