Abstract
The primary cause of the intense immune response in sarcoidosis is unclear. Potentially, a functional abnormality in dendritic cells (DCs) could cause a reduction in clearance of antigen and downstream persistence in immune activity. In this study, we investigate the interaction between monocyte-derived dendritic cells and T cells in patients with sarcoidosis compared to normal controls (n = 8 each) by examining the kinetics of autologous and allogeneic mixed leucocyte reactions over 9–10 days. We found markedly depressed proliferation kinetics in autologous DC-peripheral blood mononuclear cell (PBMC) co-cultures from sarcoid patients compared to normal subjects. In allogeneic experiments PBMCs from patients showed a reduced response to allogeneic DCs from a single donor, but no difference was observed in the ability of patients and control DCs to stimulate proliferation of allogeneic PBMC from a single donor. We conclude that there is a markedly impaired autologous mixed leucocyte reaction (MLR) in sarcoidosis patients. In allogeneic MLR, monocyte-derived DCs in sarcoidosis were able to stimulate T cells normally, but PBMCs responses were reduced. This contradicts recent published studies on ex vivo isolated myeloid DCs from sarcoidosis patients although, potentially, an in vivo conditioning factor, which reduces DC function in sarcoidosis, could be a unifying explanation for the contrasting findings.
Keywords: dendritic cells, sarcoidosis, T cells
Introduction
Sarcoidosis is a granulomatous disease of unknown aetiology, characterized by an active CD4 T helper type 1 (Th1) cell response to an undefined antigen [1]. The primary defect in this condition is unclear – both CD4 T cells and macrophages are activated, and the characteristic granuloma is likely to be an end-product of both these processes. The condition is accompanied by an unexplained, paradoxical cutaneous anergy due to a depressed type IV delayed hypersensitivity response to recall antigens such as tuberculin. This occurs in spite of intense T cell response in the lungs [1,2]. A possible cause for these observations is a defect in the antigen processing, T cell priming or tolerizing capacity of dendritic cells (DC). DCs are the most potent antigen-presenting cells (APCs), capable of both induction and regulation of immune responses. In the presence of danger and inflammatory signals they mature and acquire co-stimulatory signals to induce activation of naive antigen-specific T cells [3]. They are also involved in preventing self-destructive autoimmunity and mediate this by promoting the induction of T cell anergy, apoptosis or ignorance via their tolerogenic DC subset [4]. A functional abnormality in one or more of these aspects of DC biology could contribute to abnormalities in activation of T cells, decreased clearance of antigens and subsequent persistence in immune activity. Almost nothing has been published on DC function in sarcoid patients until a very recent paper by Mathew et al.[5] (discussed later). We have examined previously the proportion of circulating CD3-, CD14-, CD19- (lineage negative), CD11c+ cells in unenriched peripheral blood mononuclear cells (PBMC) [6] and found no difference in their frequencies compared to normal. Using absolute counts, Ota et al. showed that circulating myeloid DCs were reduced in patients, and suggested a migration of these cells to the site of inflammation [7]. In the lungs, a recent study showed an increase in the proportion of CD1a- myeloid DCs in the bronchoalveolar lavage, in contrast to patients with pneumonia and idiopathic pulmonary fibrosis [8]. These studies suggest the involvement of DCs in the immunopathology of sarcoidosis, but very little is known of their ability to stimulate or regulate T cells. We were interested in the functional aspect of DCs in sarcoidosis and started by examining two related but distinct interactions between DCs and T cells. Using a widely established method of DC derivation involving in vitro differentiation of monocytes isolated from patients and controls, we first questioned if an autologous mixed leucocyte reaction (MLR), a distinct immunological phenomenon, was different compared to normal controls, and then if allogeneic MLR between DCs from sarcoid patients and donor PBMC and T cells were abnormal.
Methods
Patients
All patients had biopsy-proven diagnosis, made in accordance with the World Association of Sarcoidosis and Other Granulomatous Disorders/American Thoracic Society (WASOG/ATS) criteria [9], were on no treatment and had pulmonary sarcoidosis with only one other system involvement (eye or skin). None presented with Loefgren's syndrome, and all were never-smokers. Eight patients (median age 43 years, range 33–58 years, six females) and eight controls, matched for age, gender and smoking history were recruited from the Oxford Sarcoidosis Clinic. All patients had minor symptoms of cough, not requiring oral corticosteroid treatment, although three were on inhaled budesonide. All patients gave informed consent and the study was approved by the Central Oxfordshire Research Ethics Committee.
PBMC, DC and T cell preparation and MLR experimental conditions
PBMC were derived by Ficoll-Hypaque density centrifugation and used on the day of isolation. Monocyte-derived DCs were prepared as described previously [6]. Briefly, CD14+ cells were derived with MACS® beads (Miltenyi Biotec, Bergisch Gladbach, Germany) and cultured in medium supplemented with 1% pooled human serum (National Blood Service, Bristol, UK), 100 ng/ml of interleukin (IL)-4 (PeproTech, Rocky Hill, NJ, USA) and 100 ng/ml of granulocyte–macrophage stimulating factor (Leucomax®, Schering-Plough, Welwyn Garden City, UK) for 5–6 days. Where appropriate, DCs were matured with 100 ng/ml Salmonella typhimurium lipopolysaccharide (LPS) (Sigma, Poole, UK) for 48 h. All DCs were checked for differentiation and maturation markers [CD83, CD86, human leucocyte antigen D-related (HLA DR)] and determined to be CD1b- and CD1d-positive and CD14-negative at time of use.
For the MLRs, 5000 monocyte-derived DCs were co-cultured with 1 × 105 autologous PBMC in culture media supplemented with human antibody serum from one batch of donors (First Link, Birmingham, UK). Proliferation levels were measured on days 3, 5 and 7 of the co-culture with the addition of 0·5 µCi of tritiated thymidine [3H] (Amersham Pharmacia, Little Chalfont, UK) in the last 16 h of the co-culture. Incorporated [3H]-thymidine was measured with a microplate beta counter (TopCount-NXT®; Packard Bioscience, Meriden, CT, USA). PBMCs and monocyte-derived DCs were isolated from eight patients with sarcoidosis; each co-culture experiment was paired with a normal subject, and fresh cells were used.
Flow cytometric studies
Flow cytometry studies of PBMC were performed on stored samples and at least 0·5 × 106 PBMCs were acquired for fluorescence activated cell sorter (FACS) analysis. All samples were analysed with a FACSCalibur (BD Biosciences, Oxford, UK) and CellQuest® software. All monoclonal antibodies were obtained from BD Biosciences.
Results
Sarcoidosis patients have decreased autologous MLR
We found a markedly depressed PBMC response to autologous DCs in patients with sarcoidosis (Fig. 1a) when both immature and mature DCs were used (Fig. 1b and c). In every paired (normal and sarcoidosis) autologous MLR experiment, cellular proliferation was lower in sarcoid patients. Peak level of proliferation was observed on days 5 or 7 of co-culture.
Fig. 1.
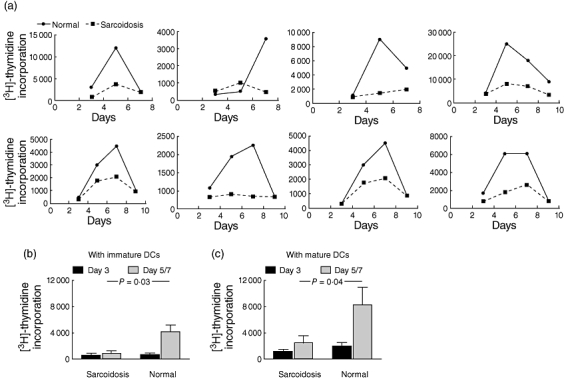
(a) Proliferation kinetics of autologous mixed leucocyte reactions (MLR) [dendritic cell–peripheral blood mononuclear cell (DC-PBMC) co-cultures] in eight pairs of sarcoid patients and normal controls at three to four time-points during a 7–9-day assay. Pairs were performed on the same day. Data for immature DCs are shown. (b) Comparing peak proliferation (days 5 or 7) between sarcoid patients and normal subjects, there was a significantly decreased level of proliferation in sarcoid patients (P-value derived from Student's t-test; mean ± standard error of the mean shown) when immature and mature autologous DCs were used.
Allogeneic MLR suggests that patients' leucocytes were unable to respond to DCs
In order to determine whether patients' DCs were unable to prime T cells, or their PBMCs were unable to respond to DCs, we examined the allogeneic response of PBMCs from the same cohort of patient and normal controls (n = 7 each; one was excluded as he was commenced on prednisolone) to immature and mature DCs derived from one normal donor, and compared this to the allogeneic response induced when DCs derived from these patients and controls were co-cultured with PBMC from one donor. We found no difference in the ability of patient and normal DCs to cause PBMC proliferation, but PBMC from sarcoid patients did not respond as well as normal PBMC to DCs from one donor (Fig. 2). This suggests that the ability of DCs to prime T cells in sarcoid patients is normal, while an abnormality in the PBMCs of sarcoid patients renders them less able to proliferate in the presence of allogeneic DCs.
Fig. 2.
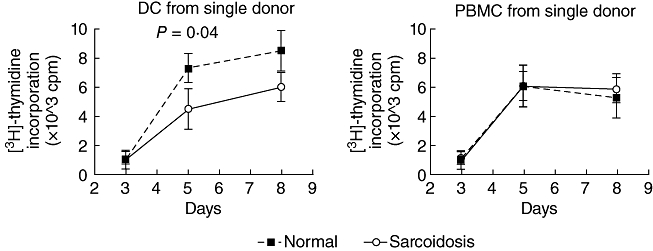
Allogeneic mixed leucocyte reactions (MLR) in seven normal and seven sarcoid patients using peripheral blood mononuclear cells (PBMC) from patients and controls co-cultured with immature dendritic cells (DC) from one donor (left panel) and immature DCs from patients and controls, each co-cultured with PBMC from one donor (right panel). Broken line refers to sarcoid patients. P-value refers to difference on day 5 (Student's t-test). All other points did not reach statistical significance.
Composition of PBMC in patients with sarcoidosis compared to normal controls
Because the numbers of PBMCs were normalized for each MLR experiment, we examined the composition of T cells in the sarcoid and normal PBMCs used in the MLR in order to determine if abnormal T cell subsets could account for the lack of response to DCs. We examined the proportion of total CD3+ cells, CD4 : CD8 ratio, central memory CD4 T cells (CD4+CCR7+CD62l+CD45RO+), effector memory CD4 T cells (CD4+CCR7-CD62l-CD45RO+) [10], naive T cells (CD3+CD45RA+) and memory T cells (CD3+CD45RO+). We found a significantly reduced proportion of effector memory T cells in our sarcoidosis cohort (Fig. 3), but no other significant differences in the other groups of T cells.
Fig. 3.
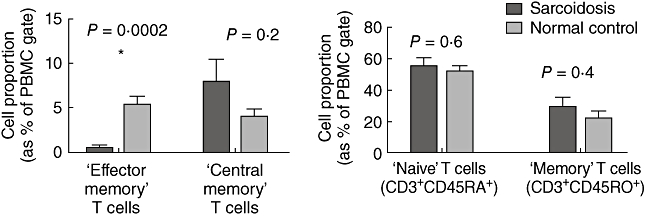
Composition of peripheral blood mononuclear cells (PBMC) in the seven normal and seven sarcoid patients used in the allogeneic peripheral blood mononuclear cell mixed leucocyte reaction (MLR) co-culture experiments in Fig. 2. ‘Effector memory’ T cells refer to CD4+CCR7+CD62l+CD45RO+ cells and ‘central memory’ T cells refer to CD4+CCR7-CD62l-CD45RO+ cells.
Discussion
Our study demonstrates two separate but related findings in DC function in sarcoidosis. First, autologous MLR is impaired markedly in patients with sarcoidosis. Autologous MLR is an old and widely reported phenomenon, distinct from allogeneic MLR, and refers to the in vitro proliferation of T cells in response to stimulation by autologous non-T cells. The stimulating cells appear to be the unique property of DC [11]. In 2001, using synapse imaging, calcium fluxes and intracellular signalling, two papers confirmed the ability of DCs to signal to naive T cells without requirement for exogenous antigen [12,13]. The dominant proliferating cell in this co-culture was shown to be a CD4 CD45RA+ T cell subset [14,15]. The function of this self-antigen-driven activity is unclear; it could provide continuous stimulation of T cells, in order to maintain a naive pool of T cells. However, its impairment has been reported in several diseases associated with dysfunction of the immune system [16–18] and is thought to reflect its role in immunoregulation and peripheral tolerance. Unlike allogeneic T cell responses, a distinct requirement for this activity is the expression of CD80 (B7·1) molecules on DCs, which appears to be more specific than the requirement for CD86 expression [19,20]. This is especially interesting in sarcoidosis given the recent finding that a functional mutation in butyrophilin-2 (BTNL-2), a B7-like molecule, is linked to sarcoidosis [21]. BTNL-2 is expressed on gut epithelium and DC-like cells, and required for proliferation of CD4 T cells in mice [19]. This marked reduction of autologous MLR could reflect a functional abnormality in the BTNL-2 (B7) pathway between DCs and T cells. One intriguing implication is that, in these patients, autologous DCs are unable to maintain an adequate pool of some T cells with immunoregulatory functions, such as invariant natural killer (iNKT) cells, which we have shown to be reduced in sarcoid patients [6]. Invariant NKT cells express CD69 constitutively and are thought to be stimulated persistently by self-antigen presentation by CD1d expressing APC-like DCs to maintain an active phenotype [22]. This fits with our and other workers' findings of reduction in both autologous MLR and the frequency of iNKT cells in sarcoidosis, and other immune-mediated diseases such as systemic lupus erythematosus (SLE) and Sjogren's syndrome [6,16–18,23,24].
In the allogeneic studies, PBMCs from sarcoid patients were not as responsive to DCs as those from normal controls. This could be because of abnormalities in the quality or composition of T cells in sarcoid PBMCs. We found a reduced frequency of effector memory T cells reflecting potential compartmentalization of these cells in lymph glands or lungs, which could explain the reduction in PBMC proliferative response. Effector memory T cells are memory cells that have lost the constitutive expression of CCR7, are heterogeneous for CD62L expression (lymphoid homing receptors) and display characteristic sets of chemokine receptors and adhesion molecules that are required for homing to inflamed tissues [10]. When compared with central memory T cells, they have more rapid effector function but reduced proliferative capacity [10,25]. Therefore, the reduction of this cell group, although in keeping with the presence of disease, cannot explain the reduction in proliferative capacity of PBMC in patients with sarcoidosis. This raises the question of a suppressive factor such as forkhead box P3-positive (FoxP3+) regulatory T cells (Tregs). Miyara and colleagues observed that FoxP3+ Tregs were increased in the circulating blood and bronchoalveolar lavage of patients with sarcoidosis and showed that Tregs derived from sarcoid patients exerted a profound anti-T cell proliferative activity [26] and a modest inhibition of the first steps of granulomagenesis in vitro.
Our findings from allogeneic MLR experiments contrast with a recent paper by Mathew and colleagues, who found that myeloid DCs derived ex vivo from patients had a poorer capacity to stimulate PBMCs from a donor, but PBMC from patients responded normally to DC from a normal donor [5]. Several explanations are possible for this difference in observation. Mathew et al. used ex vivo-derived DCs, while we used DCs that were differentiated from monocytes in vitro. An in vivo factor might condition myeloid DCs or monocytes in sarcoid patients to render them less likely to stimulate T cells. There is also another difference in the two studies that could explain the disparity. We used an MLR assay based on three time-points to allow determination of kinetics over 8–9 days, while Mathew et al. used a single time-point of day 5. Our studies in both autologous and allogeneic MLR showed that the kinetics of proliferation is different in different responders (Fig. 1a). Both days 5 and 7 could be the peak of response for different subjects. This means that the difference noted on day 5 by Mathew could reflect relative difference in kinetics between sarcoid and normal proliferation, rather than a true difference at that time-point.
In conclusion, our study shows that autologous MLR is defective in sarcoidosis. The cause of this is currently unclear but is in keeping with observations in other immune-mediated diseases and, intriguingly, occurs in association with reduction in iNKT cells. In our hands, allogeneic MLR using monocyte-derived DC showed that these DCs have the same capacity to prime T cell proliferation as those from normal subjects. Taken together with Mathew's recent study, one inference is that conditioning signals in vivo could have an effect on the function of myeloid DCs in these patients.
Disclosure
Nothing to disclose.
References
- 1.Gerke AK, Hunninghake G. The immunology of sarcoidosis. Clin Chest Med. 2008;29:379–90. doi: 10.1016/j.ccm.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 2.Daniele RP, Dauber JH, Rossman MD. Immunologic abnormalities in sarcoidosis. Ann Intern Med. 1980;92:406–16. doi: 10.7326/0003-4819-92-3-406. [DOI] [PubMed] [Google Scholar]
- 3.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–26. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 4.Hadeiba H, Sato T, Habtezion A, Oderup C, Pan J, Butcher EC. CCR9 expression defines tolerogenic plasmacytoid dendritic cells able to suppress acute graft-versus-host disease. Nat Immunol. 2008;9:1253–60. doi: 10.1038/ni.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathew S, Bauer KL, Fischoeder A, Bhardwaj N, Oliver SJ. The anergic state in sarcoidosis is associated with diminished dendritic cell function. J Immunol. 2008;181:746–55. doi: 10.4049/jimmunol.181.1.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ho LP, Urban BC, Thickett DR, Davies RJ, McMichael AJ. Deficiency of a subset of T-cells with immunoregulatory properties in sarcoidosis. Lancet. 2005;365:1062–72. doi: 10.1016/S0140-6736(05)71143-0. [DOI] [PubMed] [Google Scholar]
- 7.Ota M, Amakawa R, Uehira K, et al. Involvement of dendritic cells in sarcoidosis. Thorax. 2004;59:408–13. doi: 10.1136/thx.2003.006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lommatzsch M, Bratke K, Bier A, et al. Airway dendritic cell phenotypes in inflammatory diseases of the human lung. Eur Respir J. 2007;30:878–86. doi: 10.1183/09031936.00036307. [DOI] [PubMed] [Google Scholar]
- 9.Statement on sarcoidosis. Am J Respir Crit Care Med. 1999;160:736–55. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 10.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 11.Inaba K, Romani N, Steinman RM. An antigen-independent contact mechanism as an early step in T cell-proliferative responses to dendritic cells. J Exp Med. 1989;170:527–42. doi: 10.1084/jem.170.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Revy P, Sospedra M, Barbour B, Trautmann A. Functional antigen-independent synapses formed between T cells and dendritic cells. Nat Immunol. 2001;2:925–31. doi: 10.1038/ni713. [DOI] [PubMed] [Google Scholar]
- 13.Kondo T, Cortese I, Markovic-Plese S, et al. Dendritic cells signal T cells in the absence of exogenous antigen. Nat Immunol. 2001;2:932–8. doi: 10.1038/ni711. [DOI] [PubMed] [Google Scholar]
- 14.Karsh J, Harley JB, Goldstein R, Lazarovits AI. Ro/SSA inhibits the autologous mixed lymphocyte reaction. Clin Exp Immunol. 1993;91:103–9. doi: 10.1111/j.1365-2249.1993.tb03362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takeuchi T, Rudd CE, Schlossman SF, Morimoto C. Induction of suppression following autologous mixed lymphocyte reaction; role of a novel 2H4 antigen. Eur J Immunol. 1987;17:97–103. doi: 10.1002/eji.1830170117. [DOI] [PubMed] [Google Scholar]
- 16.Sakane T, Steinberg AD, Green I. Failure of autologous mixed lymphocyte reactions between T and non-T cells in patients with systemic lupus erythematosus. Proc Natl Acad Sci USA. 1978;75:3464–8. doi: 10.1073/pnas.75.7.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyasaka N, Sauvezie B, Pierce DA, Daniels TE, Talal N. Decreased autologous mixed lymphocyte reaction in Sjögren's syndrome. J Clin Invest. 1980;66:928–33. doi: 10.1172/JCI109960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keystone EC, Poplonski L, Snow KM, Martell M. Impaired autologous mixed lymphocyte reaction (AMLR) reactivity of peripheral blood T cell subsets in rheumatoid arthritis. Clin Exp Immunol. 1989;78:184–8. [PMC free article] [PubMed] [Google Scholar]
- 19.Ferlazzo G, Semino C, Meta M, Procopio F, Morandi B, Melioli G. T lymphocytes express B7 family molecules following interaction with dendritic cells and acquire bystander costimulatory properties. Eur J Immunol. 2002;32:3092–101. doi: 10.1002/1521-4141(200211)32:11<3092::AID-IMMU3092>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 20.Scheinecker C, Machold KP, Majdic O, Höcker P, Knapp W, Smolen JS. Initiation of the autologous mixed lymphocyte reaction requires the expression of costimulatory molecules B7-1 and B7-2 on human peripheral blood dendritic cells. J Immunol. 1998;161:3966–73. [PubMed] [Google Scholar]
- 21.Valentonyte R, Hampe J, Huse K, et al. Sarcoidosis is associated with a truncating splice site mutation in BTNL2. Nat Genet. 2005;37:357–64. doi: 10.1038/ng1519. [DOI] [PubMed] [Google Scholar]
- 22.Matsuda JL, Mallevaey T, Scott-Browne J, Gapin L. CD1d-restricted iNKT cells, the ‘Swiss-Army knife’ of the immune system. Curr Opin Immunol. 2008;20:358–68. doi: 10.1016/j.coi.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kojo S, Adachi Y, Keino H, Taniguchi M, Sumida T. Dysfunction of T cell receptor AV24AJ18+, BV11+ double-negative regulatory natural killer T cells in autoimmune diseases. Arthritis Rheum. 2001;44:1127–38. doi: 10.1002/1529-0131(200105)44:5<1127::AID-ANR194>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 24.van der Vliet HJ, von Blomberg BM, Nishi N, et al. Circulating V24+Vβ11+ NKT cell numbers are decreased in a wide variety of diseases that are characterized by autoreactive tissue damage. Clin Immunol. 2001;100:144–8. doi: 10.1006/clim.2001.5060. [DOI] [PubMed] [Google Scholar]
- 25.Geginat J, Lanzavecchia A, Sallusto F. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood. 2003;101:4260–6. doi: 10.1182/blood-2002-11-3577. [DOI] [PubMed] [Google Scholar]
- 26.Miyara M, Amoura Z, Parizot C, et al. The immune paradox of sarcoidosis and regulatory T cells. J Exp Med. 2006;203:359–70. doi: 10.1084/jem.20050648. [DOI] [PMC free article] [PubMed] [Google Scholar]


