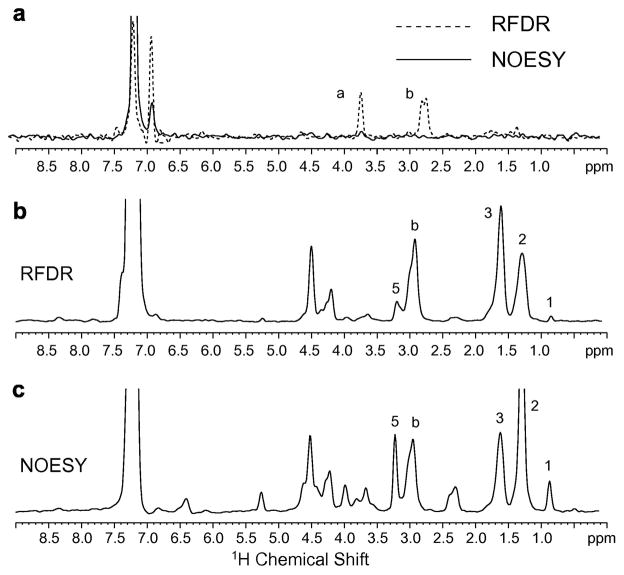
Fig. 9.
Comparison of 1H MAS NOESY and 1H MAS RFDR spectra of Ac-KKKFSFKKK-OMe. (a) Peptide bound to deuterated (D67) DMPC bilayers. Rows through the diagonal resonance of the aromatic phenylalanine ring proton resonance at 7.11 ppm are shown comparing 1H MAS NOESY (solid line) with 1H MAS RFDR (dashed line) for the same mixing time (50 msec) and number of scans. The intramolecular crosspeaks to the Cα-H and CβH protons of phenylalanine dominate the 1H MAS RFDR spectrum. The peptide-to-lipid molar ratio was 1:20. (b) Peptide bound to DMPC:DMPG bilayers using 1H MAS RFDR with a mixing time of 50 msec. The row through the diagonal resonance of the aromatic phenylalanine ring protons at 7.11 ppm shows the correlations to the lipid protons. (c) Peptide bound to DMPC:DMPG bilayers using 1H MAS NOESY with a mixing time of 300 ms. The same row as in (b) is shown through the diagonal resonance of the aromatic phenylalanine ring protons at 7.11 ppm.