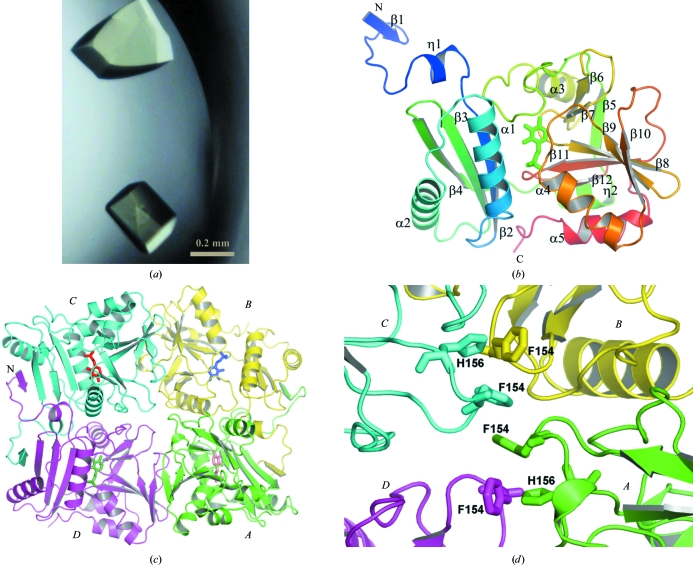
Figure 1.
The structure of TTHA0621. (a) Crystals of the TTHA0621 protein. (b) Ribbon diagram of the tertiary structure of TTHA0621 (coloured in a rainbow ramp from blue at the N-terminus to red at the C-terminus). The PLP cofactor molecule is shown as a stick model. (c) Ribbon diagram of the quaternary structure of the TTHA0621 tetramer (shown in an arbitrary orientation) coloured by subunit. The N-terminal β-strands β1 of subunits A and D and those of their intact dimer subunits, B and C, respectively, form intermolecular antiparallel β-sheets in their respective dimers. Subunits A, B, C and D are coloured green, yellow, cyan and magenta, respectively. The PLP cofactor molecule is shown as a stick model. (d) Near the tetramer region. The weak tetramerization occurs through hydrophobic interactions between the four subunits (A–D) contributed by Phe154 and His156, which are shown as stick models. All figures were produced with PyMOL (DeLano, 2002 ▶) unless mentioned otherwise.