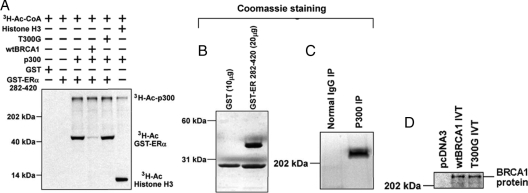
Figure 6.
BRCA1 inhibits in vitro acetylation of ER-α by p300. A, In vitro acetylation assays were carried out as described in Materials and Methods. These assays test the ability of p300 (generated from a p300 immunoprecipitation) to acetylate a GST-ER-α (amino acids 282-420) chimeric protein using [3H]acetyl coenzyme A as the substrate. Assay reactions were carried out in the absence or presence of in vitro-translated (IVT) wtBRCA1 or mutant full-length BRCA1 (T300G). Histone H3 was used as a positive control substrate for acetylation by p300. Bands corresponding to autoacetylated p300 (3H-Ac-p300), acetylated GST-ER 282-420 (3H-Ac-GST-ERα), and acetylated histone (3H-Ac-Histone H3) are indicated. B–D, The GST-ER 282-420 (B) and immunoprecipitated (IP) p300 proteins (C) were analyzed by SDS-PAGE and detected by Coomassie staining; D Western blot showing the IVT wtBRCA1 and BRCA1-T300G proteins (0.5 μg). As a negative control, IVT was carried out using an empty pcDNA3 vector.