Abstract
Background and Aims
Crown structure and above-ground biomass investment was studied in relation to light interception of trees and lianas growing in a 6-month-old regenerating forest.
Methods
The vertical distribution of total above-ground biomass, height, diameter, stem density, leaf angles and crown depth were measured for individual plants of three short-lived pioneers (SLPs), four long-lived pioneers (LLPs) and three lianas. Daily light interception per individual Φd was calculated with a canopy model. The model was then used to estimate light interception per unit of leaf mass (Φleaf mass), total above-ground mass (Φmass) and crown structure efficiency (Ea, the ratio of absorbed vs. available light).
Key Results
The SLPs Trema and Ochroma intercepted higher amounts of light per unit leaf mass (Φleaf mass) because they had shallower crowns, resulting in higher crown use efficiency (Ea) than the other species. These SLPs (but not Cecropia) were also taller and intercepted more light per unit leaf area (Φarea). LLPs and lianas had considerably higher amounts of leaf mass and area per unit above-ground mass (LMR and LAR, respectively) and thus attained Φmass values similar to the SLPs (Φmass=Φarea×LAR). Lianas, which were mostly self-supporting, had light interception efficiencies similar to those of the trees.
Conclusions
These results show how, due to the trade-off between crown structure and biomass allocation, SLPs, and LLPs and lianas intercept similar amount of light per unit mass which may contribute to the ability of the latter two groups to persist.
Key words: Bolivia, canopy model, crown structure, leaf mass ratio, lianas, light interception, pioneers, specific leaf area, tropical forest
INTRODUCTION
Abandoned agricultural fields in tropical forest areas are typically colonized by pioneer trees and lianas. Along the spectrum of life histories and ecological requirements pioneer trees are broadly classified into short- and long-lived species (Whitmore, 1989; Finegan, 1996). In fallowed fields SLPs rapidly develop a closed canopy and dominate for about 10–30 years, with their peak in abundance during the first 2–4 years of succession (Peña-Claros, 2003). Long-lived pioneers (LLPs) are already present in the first year of succession but initially occupy lower layers of the canopy and become dominant later on (Finegan, 1996; Peña-Claros, 2003). While lianas also occur in mature forest, their peak in abundance is also early in succession (De Walt et al., 2000; Schnitzer and Bongers, 2002; Gerwing, 2004). Interestingly, most lianas go through a self-supporting seedling phase (Putz, 1984; Caballe, 1998), although the height attained by self-supporting individuals varies among species (Putz, 1984). But, how does the seedling growth habit of lianas enable them to compete with trees? Moreover, what characteristics enable SLPs to achieve early dominance and how are LLPs able to persist below the short-lived ones early in succession?
During the early stages of secondary forest succession, standing biomass rapidly increases. Given this condition, it follows that a strong vertical light gradient is created, along which some trees grow in the shade of others. Competing for light probably plays an important role in determining the course of succession (Werger et al., 2002). Many studies have compared light requirement for growth and survival between pioneers and late-successional shade-tolerant species (Pompa and Bongers, 1988; Chazdon, 1992; King, 1994; Kitajima, 1994; Veneklaas and Poorter, 1998; Valladares et al., 2000), but relatively few have compared this between different pioneer species (but see Peña-Claros, 2001; Dalling et al., 2004; Poorter et al., 2006).
Most studies that attempted to relate individual traits of different tropical forest tree species to their ability to capture light, focused on interspecific differences in crown characteristics and leaf morphologies (King, 1994; Kitajima, 1994; Poorter, 1999; Valladares, 2002; Falster and Westoby, 2003; Poorter et al., 2006). Crown structure is obviously an important determinant of light capture (Horn, 1971) and tropical species show a large variation in leaf morphology and crown structure (Bongers and Popma, 1990; Valladares et al., 2002; Poorter et al., 2006). Based on the concept of optimal crown structure for maximum light capture several researchers predicted that shade-tolerant trees should have broad crowns with little leaf overlap to minimize self-shading, whereas light-demanding trees should have narrow crowns with numerous leaf layers (Horn, 1971; Kohyama, 1987; King, 1990). These predictions have not been confirmed in the field; generally crowns of shade-tolerant trees were deeper with more leaf layers than those of the pioneer trees (e.g. Poorter, 1998; Sterck et al., 2001; Kitajima et al., 2005). The production of a broad crown with minimal self-shading while maintaining an efficient angular leaf display with respect to the prevailing light direction requires substantial investment in support (additional branches), which might be prohibitively expensive for trees growing under shaded conditions (Valladares et al., 2002). For light-demanding species, investing biomass for height growth improves access to light, but comes at the expense of foliage and branch growth and may require continuous remobilization of resources from older to younger leaves (Smith, 1982; King, 1994; Gilbert et al., 2001). For these reasons, such species would be expected to have shallower crowns.
Models that include only crown geometry or leaf morphology are apparently not able to predict species competitive interactions in tropical forests and thus there is a need for a more integrated approach that considers biomass expenditure. Hirose and Werger (1995) developed such an approach that relates biomass allocation patterns and crown structure with light interception and then applied this method to dense temperate grassland vegetation (Hirose and Werger, 1995). They calculated that tall dominant species absorbed more light per unit of leaf area [Φarea, the PPFD (photosynthetic photon flux density) captured per unit of leaf area] than subordinate ones. Surprisingly, the amount of PPFD captured per unit of above-ground mass (Φmass) by subordinate species was similar or higher than that of the tall dominant species. With this approach they demonstrated that during succession in grasslands, early dominance is closely associated with high rates of stem elongation and internode length, while persistence at low irradiance was associated with high specific leaf area (SLA) and comparatively high Φmass (Werger et al., 2002; Anten, 2005; Hirose, 2005).
As noted, these studies were conducted in grasslands where above-ground mass accumulated for only one season. In contrast, in woody vegetation, above-ground mass accumulates continuously for many years. Trade-offs related to crown structure and biomass investments for light interception that enables some species to attain dominance and to others to coexist lower in the canopy may therefore be different in herbaceous than in woody vegetation.
We predict that SLPs possess biomass allocation traits that facilitate high daily light interception per unit mass (Φmass) relative to LLPs. The lianas, while self-supporting very early in succession, will eventually start climbing relying on other plants for support. Consequently, they may not have to invest as much in a durable support structure as trees. We predict that they will allocate more mass to leaves thus achieving higher Φmass values. To test these hypotheses, a grassland canopy model (Hirose and Werger, 1995; Anten and Hirose, 1999) was modified to incorporate specific features of forest trees. In combination with field measurements this model made it possible to relate interspecific differences in biomass allocation and crown structure to light interception. The focus on a very young stand (6 months since cessation of agricultural activity) because at this stage individuals of the three different groups are still not very different in size, and small differences in light capture and height growth greatly influence species composition and size hierarchies in the subsequent 5–10 years.
MATERIALS AND METHODS
Study site and plant material
A secondary forest stand growing near Riberalta in the Bolivian Amazon (11°S 66·1°W) was studied. The area had been slashed and burned, cropped with rice, maize and cassava in a sequence of three years and then abandoned. The study was conducted 6 months after land abandonment. A plot of approx. 0·7 ha was selected for the study. The plot was located at a distance of at least 20 m from the edge of the stand to avoid the influence of the surrounding vegetation. The area was surrounded by old growth forest.
In the regrowing vegetation, which formed a very homogenous canopy, ten of the most common species were selected based on a previous study on species diversity and abundance along a chronosequence (Peña-Claros, 2003; N. G. Selaya, unpubl. res.) Trema micrantha, Ochroma pyramidale and Cecropia ficifolia are present from the time of land abandonment to 4–25 years later and denoted as short-lived pioneers (SLPs). Couratari guianensis, Rinoreocarpus uleii, Pseudolmedia laevis and Brosimum lactescens are found from land abandonment to old growth forest, with a peak in abundance between 30 and 100 years, and are thus referred to those as LLPs. The lianas Uncaria guianensis, Hippocrateaceae species and Bignoniaceae species are present from early stages of succession and persist till the old growth forest and in this study they are treated as a separate group due to the climbing growth habit they develop later in life. In the study field, however, almost all liana individuals were still self-supporting. Hereafter species are named by generic (or family) names. Ten to twenty individuals of different heights per species were selected such that they covered the height range with which each species occurred in the 6-month-old stand. All individuals had grown from seed. Resprouts were carefully avoided as these may have a different carbon balance than seedlings.
Canopy structure and light (PPFD) distribution
Canopy structure and light distribution were determined in October, at the beginning of the rainy season. The main plot was subdivided into 63 subplots of 9 m2 each. Individuals were selected inside the subplots. PPPFD (400–700 nm) was measured at vertical increments of 25 cm in each subplot that contained at least one of the selected individuals. An SF 80 Line Sensor (Decagon devices Ltd, UK) was used to measure the PPFD in the canopy and simultaneous measurements of PPFD above the canopy were taken with a point Li-190 SA Quantum sensor (LiCor, NE, USA), connected to a data logger LI1000 (LiCor). Average leaf area index (LAI, m2 m−2) and average leaf angle distribution in each subplot were estimated with an LAI-2000 plant canopy analyser (LiCor). An above-canopy measurement followed by four below-canopy measurement, viewing from each subplot corner to the centre was taken. A view cap of 45° was used to restrict the lens field of view. PPFD and LAI measurements were taken under an overcast sky or at sunset. The vertical distribution of leaves in the canopy was measured using the point method, lifting a scaled pole from the bottom to the top of the canopy and recording the height at which the tip of the pole touched a leaf (Sterck et al., 2001). The procedure was repeated at the centre of every square metre of the 9-m2 subplot, for nine replicates per subplot.
Stem allometry, crown structure and above-ground biomass allocation
Total height, height to the first leaf or branch with leaves, stem diameter at 30 and 130 cm height were measured. When individuals were <30 cm tall, stem diameter was measured at 10 cm. Individuals were stratified into horizontal layers of 25 cm and the inclination angles of five randomly selected leaves were measured in each layer using a hand-held protractor. The distribution of the above-ground biomass was determined by destructive harvesting. Individuals were harvested and clipped into 25-cm height segments. Stems, branches, petioles and leaves were put separately in plastic bags. Digital photographs of a representative sample of leaves were taken to obtain leaf area of the individual. Leaf area was calculated using the Sigma Scan Pro 5 (SPSS Inc). Fresh material was oven dried at 70 °C for about 5 d and weighed to obtain dry mass.
For each individual the stem mass ratio (SMR, stem mass per above-ground mass, g g−1) and stem density (dry mass per volume, in g cm−3) were calculated. Stem density was estimated for segments that ranged between 30 and100 cm in length. The volume was calculated as 0·25D2πL, where L is the segment length and D the diameter measured at the middle of the segment. Crown depth (fraction of total length with leaves, as a percentage) was calculated. Leaf mass ratio (LMR, leaf mass per above-ground mass, in g g−1), specific leaf area (SLA, leaf area per leaf mass, in cm2 g−1) and leaf area ratio (LAR, leaf area per above-ground mass, in cm2 g−1) were also calculated.
Model
The model works with 9-m2 subplots to account for the vegetation heterogeneity and divides the subplots into 25-cm horizontal layers i. The subplots contain individual plants of the selected species. Two illumination classes are distinguished: shaded and sunlit leaf area (Depury and Farquhar, 1997). The PPFD (mol m−2 s−1) intercepted by the shaded leaf area of individual plants in layer i (Ishp,i) is given as:
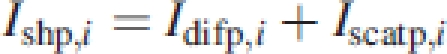 |
1 |
where Idifp,i and Iscatp,i are the diffuse-sky irradiance and the scattered-beam irradiance (light scattered by leaves in the canopy), respectively.
Idifp,i and Iscatp,i can be calculated using the approximate exponential expressions:
| 2 |
| 3 |
where Iadif is the diffuse PPFD above the canopy, kdifp and kdifveg are the extinction coefficient of diffuse light of the target plant and that of the vegetation, respectively, and Fcum the cumulative LAI above the point in layer i. Canopy reflection γ is assumed to be 5 % and the mean leaf absorbance α is assumed to be 0·8 (Goudriaan, 1977). In eqn (3) Iadir is the beam PPFD above the canopy; kblp,i and kblveg,i are the extinction coefficients for direct light of the plant and the vegetation, respectively. The model distinguishes between the extinction coefficient of the vegetation and that of individual plants within the vegetation because they may differ in leaf angle distributions. Sunlit leaves receive both direct-beam and diffuse-sky irradiance. The light intercepted by a single sunlit leaf of an individual in a canopy layer i (Islp,i) is calculated as:
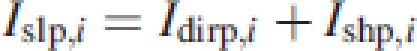 |
4 |
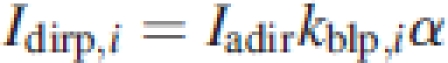 |
5 |
where Idirp,i is the direct beam and Ishp,i is the diffuse light. Iadir is the beam irradiance above the canopy, kblp,i is the coefficient of extinction of the plant, and α is the leaf absorbance. The extinction coefficients of vegetation and individuals were calculated following Goudriaan (1988) and using equations 3–5 from Anten (1997)
The light intercepted per unit of leaf area in layer i, (Ip,i, mol s−1), is the sum of light intercepted by sunlit and shaded leaf area integrated over the cumulative LAI from the top to the bottom of the layer:
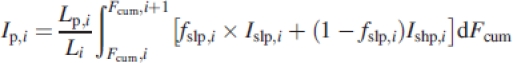 |
6 |
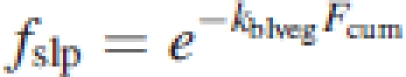 |
7 |
where Lp,i is the leaf area of an individual plant in layer i and Li is the total leaf area in that layer both in square metres, fslp,i is the fraction of sunlit leaves at a certain depth in the canopy and Ishp,i and Islp,i are the light intensity on shaded and sunlit leaves areas, respectively. The proportional LAI (Lp,i) per 25 cm vegetation layer was calculated by multiplying the proportion of touched points in that layer (determined with the point method) with the total LAI.
To obtain the daily light capture per layer Idp,i (mol plant−1 d−1), the value of Ip,i is integrated over the day from sunrise to sunset:
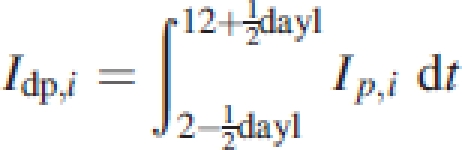 |
8 |
where, dayl is the day length on 15 October, the median day of our harvesting period and at latitude 11°S 66·1°W. The dayl and the solar inclination angle are calculated according to Gates (1980). The total daily light capture per plant Φd is calculated as:
 |
9 |
Light interception efficiencies
The daily amount of light intercepted per unit of leaf area (Φarea,), leaf mass (Φleaf mass) and total above-ground mass (Φmass), all in mol g−1 d−1, was calculated as:
 |
10 |
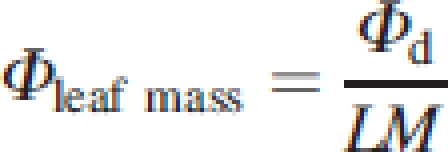 |
11 |
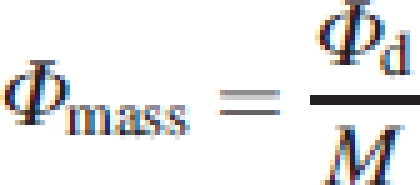 |
12 |
with A, LM and M the leaf area, leaf mass, and total above-ground mass of a plant, respectively. The relationships between plant height, crown structure and biomass allocation on the one hand and light capture per unit mass on the other can be defined as:
| 13 |
with LAR and SLA the leaf area ratio and the specific leaf area, respectively, and:
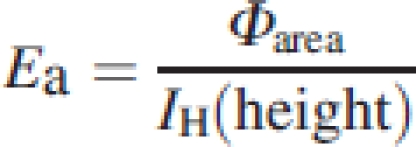 |
14 |
with Ea the crown efficiency of light absorption – the ratio of absorbed vs. available light – which is a function of leaf angles and leaf area distributions (see Valladares et al., 2002), IH(height) the total daily irradiance on a horizontal plane right above the plant. A list of symbols is given in Table 1.
Table 1.
List of the most important symbols
| Variables | Definition | Units |
|---|---|---|
| LAI | Leaf area index | m2 m−2 |
| SLA | Specific leaf area | cm2 g−1 |
| LMR | Leaf mass ratio | g g−1 |
| LAR | Leaf area ratio | cm2 g−1 |
| SMR | Stem mass ratio | g g−1 |
| PPFD | Photosynthetic photon flux density | mol m−2 s−1 |
| Φd | PPFD attained by an individual plant per day | mol plant−1 d−1 |
| Φarea | PPFD per leaf area per day | mol cm−2 d−1 |
| Φleaf mass | PPFD per leaf mass per day | mol g−1 d−1 |
| Φmass | PPFD per total above-ground mass per day | mol g−1 d−1 |
| Ea | Crown efficieny index | mol mol−1 |
Statistics
The effect of species on height, biomass, diameter, stem density, crown depth, Φd,, Φarea, Φleaf mass and Φmass were tested with ANOVA. Variables were log transformed, except LMR, to meet the assumption of homogeneity of variances of the Levene's test (P<0·05).When the results of Levene's test were significant (P<0·01) after data transformation, the ANOVA Welch test was used. Pair-wise post hoc Sidak tests were used to test differences among species and Games-Howell tests for variables with significant differences in variance. A second-order polynomial regression was done to test the linearity of the relationships between height, biomass and diameter. ANCOVA was applied with height as the dependent variable and diameter and above-ground biomass as covariates, and an ANCOVA with LMR, LAR Φarea or SLA as the dependent variable and either height or Φarea as the covariate with species as a discrete factor. The same procedure was applied for Φd, Φarea, Φleaf mass or Φmass as the dependent variable and either height or mass as covariates with species as a discrete factor.
In the regression analysis the choice of independent vs. dependent variable was based on causal relationships assumed in the model. Height and light interception were analysed as dependent variables against mass or diameter (in the case of height) as dependent, to indicate how efficient a given amount of mass is converted into height or used for light interception. LMR and LAR were analysed against height, because it was assumed that due to biomechanic constraints, as plant grow taller they have to invest desproportionally in support at the expenses of leaves. Finally, SLA was analysed relative to Φarea because SLA is usually negatively correlated with light intensity.
RESULTS
Canopy structure and light vertical distribution
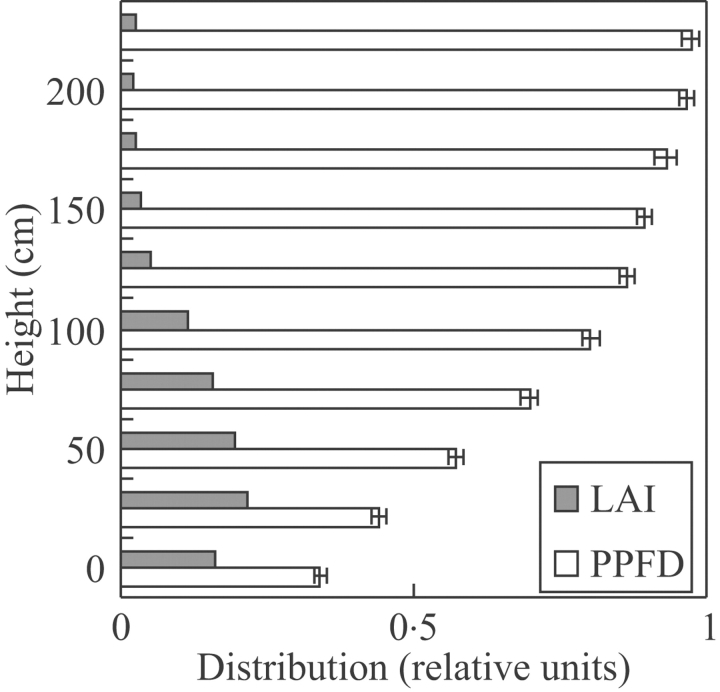
The vegetation was on average 1·8 m tall (maximum 2·25 m). The mean leaf area index (LAI) was 1·63±0·05 and tended to be concentrated between 0 and 75 cm above the ground (Fig. 1). Light (PPFD) availability at the forest floor was about 34 % and increased to 75 % at 75 cm above ground level.
Fig. 1.
Mean relative LAI and PPFD distribution as a function of height (cm) of 63 subplots in a 6-month-old regenerating tropical secondary forest stand. Bars denote standard error.
Allometry and biomass allocation
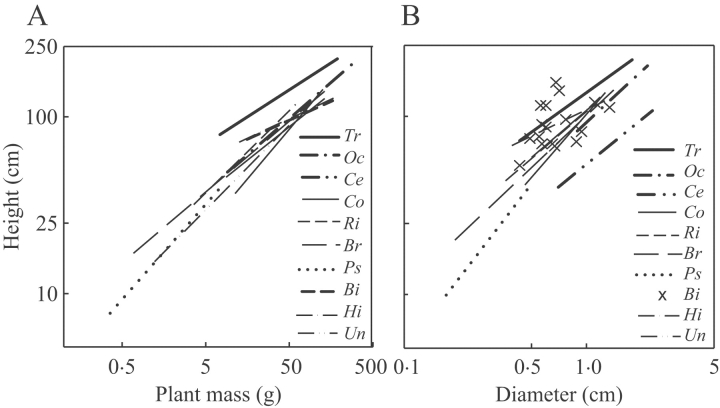
The species differed in height, total above-ground biomass, stem diameters, stem density and crown depth (Table 2). Two SLPs Trema and Ochroma were on average taller than the other species, while Ochroma plants had the highest mean above-ground mass of all species. Cecropia plants were similar in height and above-ground mass to the LLPs and lianas. Species height and above-ground mass, and height and stem diameter were positively correlated (Fig. 2A and B). Trema attained the greatest height at a given mass and stem diameter. The analysis of covariance (ANCOVA) showed heterogeneity in the slopes of the relationship between height and mass, whereas homogeneity of slopes was found for the relationship between height and diameter (Table 3). All regressions of height and mass by species were significant except for Bignoniaceae species (Table 4). The quadratic terms in the second order polynomial regression of height vs. diameter and height vs. mass was not significant (P values ranging from 0·07 to 1) indicating that these relationships were linear. The SLP species had lower stem densities and shallower crowns than the other trees (Table 2).
Table 2.
Mean plant height, above-ground mass, diameter, wood density, crown depth, SLA, LMR, LAR, Φd and Φarea of short-lived (SLPs), long-lived (LLPs) pioneer trees and lianas (L) in a 6-month-old secondary forest stand
| Species | Group | n | Height (cm) | s.e. | P<0·001 Sidak | Plant mass (g) | s.e. | P<0·001 Sidak | Diameter (cm) | s.e. | P<0·001 Sidak | Wood density (g cm−3) | s.e. | P<0·001 Sidak | Crown depth (cm cm−1) | s.e. | P<0·001 G–H |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trema | SLPs | 17 | 140·13 | 12·22 | a | 63·65 | 14·60 | abc | 1·00 | 0·10 | bc | 0·28 | 0·02 | bc | 0·65 | 0·04 | b |
| Ochroma | SLPs | 18 | 129·22 | 12·56 | a | 115·76 | 20·68 | a | 1·40 | 0·11 | a | 0·26 | 0·02 | c | 0·36 | 0·04 | c |
| Cecropia | SLPs | 17 | 73·71 | 7·31 | bcd | 38·10 | 9·02 | bc | 1·39 | 0·09 | ab | 0·17 | 0·02 | e | 0·36 | 0·04 | c |
| Couratari | LLPs | 17 | 96·59 | 8·18 | abc | 67·83 | 9·53 | ab | 0·88 | 0·07 | cd | 0·43 | 0·04 | abc | 0·85 | 0·02 | a |
| Rinereocarpus | LLPs | 16 | 79·60 | 7·76 | bcd | 27·35 | 4·03 | bc | 0·67 | 0·05 | cde | 0·42 | 0·04 | abc | 0·80 | 0·04 | ab |
| Brosimum | LLPs | 17 | 64·53 | 9·41 | cd | 29·60 | 10·05 | c | 0·63 | 0·09 | e | 0·43 | 0·07 | abc | 0·85 | 0·03 | a |
| Pseudolmedia | LLPs | 13 | 22·38 | 3·27 | e | 3·05 | 0·76 | d | 0·30 | 0·03 | f | 0·47 | 0·06 | ab | 0·78 | 0·04 | ab |
| Bignoniaceae sp. | L | 20 | 95·84 | 6·82 | abc | 51·66 | 9·00 | abc | 0·71 | 0·06 | cde | 0·54 | 0·05 | a | 0·85 | 0·02 | a |
| Hippocrateaceae sp. | L | 16 | 97·13 | 6·01 | abc | 56·85 | 10·89 | abc | 0·78 | 0·06 | cde | 0·49 | 0·04 | a | 0·86 | 0·02 | a |
| Uncaria | L | 16 | 60·13 | 10·24 | d | 32·47 | 9·87 | bc | 0·79 | 0·08 | de | 0·36 | 0·05 | abc | 0·78 | 0·03 | ab |
| Species | Group | SLA (cm2 g−1) | s.e. | P<0·001 Sidak | LMR (g g−1) | s.e. | P<0·001 Sidak | LAR (cm2 g−1) | s.e | P<0·001 G–H | Φd (mol d−1) | s.e. | P<0·001 G–H | Φarea (mol cm−2 d−1) | s.e. | P<0·001 Sidak | |
| Trema | SLPs | 188·58 | 5·55 | b | 0·36 | 0·02 | d | 68·24 | 4·96 | d | 9·35 | 2·07 | ab | 0·0024 | 0·00014 | a | |
| Ochroma | SLPs | 149·68 | 2·27 | c | 0·39 | 0·02 | d | 58·65 | 3·18 | d | 18·11 | 3·81 | a | 0·0025 | 0·00014 | a | |
| Cecropia | SLPs | 152·59 | 2·70 | c | 0·37 | 0·02 | d | 57·15 | 3·50 | d | 4·30 | 1·21 | bc | 0·0019 | 0·00012 | ab | |
| Couratari | LLPs | 121·14 | 2·27 | d | 0·61 | 0·02 | ab | 73·77 | 2·52 | cd | 9·04 | 1·52 | abc | 0·0018 | 0·00012 | bc | |
| Rinereocarpus | LLPs | 227·72 | 6·14 | a | 0·47 | 0·03 | c | 106·37 | 7·62 | ab | 5·42 | 1·27 | abc | 0·0015 | 0·00011 | cd | |
| Brosimum | LLPs | 145·37 | 5·26 | c | 0·58 | 0·02 | ab | 84·51 | 5·23 | bc | 2·74 | 0·75 | c | 0·0014 | 0·00008 | de | |
| Pseudolmedia | LLPs | 173·57 | 5·22 | b | 0·72 | 0·02 | a | 125·65 | 6·09 | a | 0·32 | 0·07 | d | 0·0009 | 0·00006 | f | |
| Bignoniaceae sp. | L | 117·27 | 3·22 | d | 0·53 | 0·02 | b | 61·43 | 2·23 | d | 5·19 | 0·78 | abc | 0·0017 | 0·00008 | bc | |
| Hippocrateaceae sp. | L | 124·64 | 3·15 | d | 0·49 | 0·02 | b | 61·58 | 3·41 | d | 6·91 | 1·71 | abc | 0·0021 | 0·00008 | ab | |
| Uncaria | L | 172·85 | 9·63 | b | 0·61 | 0·03 | ab | 107·31 | 9·69 | a | 3·66 | 1·09 | bc | 0·0013 | 0·00013 | ef | |
Significance levels (P values) of the overall species effect are shown.
Different letters indicate significant differences (P<0·05) between species pairs (test type indicated).
Fig. 2.
Regression lines per species of (A) plant height vs. above-ground mass and (B) height vs. diameter. Lines denote species with significant regression (P<0·05). Symbols denote species with no significant correlation. Abbreviations of the species: Tr, Trema; Oc, Ochroma; Ce, Cecropia – all three short-lived pioneers; Co, Couratari; Ri, Rinereocarpus; Br, Brosimum); Ps, Pseudolmedia – all four long-lived pioneers; Bi, Bignoniaceae spescies; Hi, Hippocrateaceae species; Un, Uncaria – all three lianas. Data were log-transformed.
Table 3.
Analysis of covariance (ANCOVA) of plant height, LMR (leaf mass ratio), LAR (leaf area ratio), Φd (PPFD), Φarea (PPFD per leaf area per day) and Ea (crown efficiency index), SLA (specific leaf area) as dependent variables with either plant above-ground mass, height, diameter or Φarea as covariates and species as discrete factor
| Dependent | Covariate | F values | Slope effect, P | Intercept effect, P |
|---|---|---|---|---|
| Height | Mass | F9,140=3·21 | 0·001 | n.a. |
| Height | Diameter | F9,123=1·45 | n.s. | <0·001 |
| LMR | Height | F9,140=0·76 | n.s. | <0·001 |
| LAR | Height | F9,140=1·30 | n.s. | <0·001 |
| Φd | Mass | F9,147=1·70 | n.s. | <0·001 |
| Φarea | Height | F9,147=3·18 | 0·02 | n.a. |
| Ea | Height | F9,143=5·79 | <0·001 | n.a. |
| SLA | Φarea | F9,147=2·91 | 0·03 | n.a. |
P<0·05 values are shown; n.a., not applicable; n.s., not significant.
Table 4.
Coefficients of the allometric relationships between plant height and above-ground mass, height and diameter, LMR and height, LAR and height, and Φd and height, and SLA and Φarea (i.e. ln height=a+b log mass)
| Species | Group | Height vs. mass |
Height vs. diameter |
LMR vs. height |
LAR vs. height |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Constant (a) | Slope (b) | P value | r2 | Constant (a) | Slope (b) | P value | r2 | Constant (a) | Slope (b) | P value | r2 | Constant (a) | Slope (b) | P value | r2 | ||
| Trema | SLPs | 3·76 | 0·30 | <0·001 | 0·83 | 4·92 | 0·70 | <0·001 | 0·82 | 0·56 | −0·36 | 0·02 | 0·33 | 5·65 | −0·38 | 0·018 | 0·32 |
| Ochroma | SLPs | 2·98 | 0·40 | <0·001 | 0·87 | 4·53 | 0·95 | <0·001 | 0·42 | 0·46 | −0·30 | 0·04 | 0·20 | 5·33 | −0·27 | 0·06 | 0·23 |
| Cecropia | SLPs | 2·99 | 0·38 | <0·001 | 0·89 | 3·99 | 0·83 | 0·01 | 0·40 | 0·62 | −0·39 | 0·03 | 0·28 | 5·66 | −0·39 | 0·02 | 0·41 |
| Couratari | LLPs | 2·24 | 0·56 | <0·001 | 0·86 | 4·63 | 1·17 | <0·001 | 0·64 | 0·37 | −0·19 | <0·001 | 0·65 | 5·21 | −0·21 | 0·002 | 0·43 |
| Rinereocarpus | LLPs | 2·86 | 0·46 | <0·001 | 0·74 | 4·67 | 0·82 | 0·02 | 0·36 | −0·66 | −0·03 | 0·87 | 0·002 | 5·19 | −0·13 | 0·51 | 0·04 |
| Brosimum | LLPs | 2·98 | 0·40 | <0·001 | 0·83 | 4·62 | 0·97 | 0·001 | 0·64 | 0·13 | −0·17 | <0·001 | 0·69 | 5·58 | −0·29 | <0·001 | 0·59 |
| Pseudolmedia | LLPs | 2·60 | 0·54 | <0·001 | 0·96 | 4·64 | 1·33 | 0·001 | 0·84 | −0·002 | −0·11 | 0·04 | 0·33 | 5·21 | −0·13 | 0·16 | 0·21 |
| Bignoniaceae sp. | L | 3·72 | 0·21 | 0·05 | 0·21 | 4·65 | 0·39 | 0·15 | 0·11 | −0·507 | −0·03 | 0·81 | 0·0004 | 4·54 | −0·09 | 0·50 | 0·01 |
| Hippocrateaceae sp. | L | 3·72 | 0·22 | 0·003 | 0·49 | 4·69 | 0·49 | 0·01 | 0·38 | 0·83 | −0·34 | 0·04 | 0·28 | 6·43 | −0·51 | 0·02 | 0·40 |
| Uncaria | L | 2·62 | 0·48 | <0·001 | 0·84 | 4·68 | 0·87 | 0·001 | 0·63 | 0·16 | −0·18 | <0·001 | 0·83 | 6·13 | −0·40 | <0·001 | 0·83 |
| Species | Group | Φdvs. mass | Φareavs. height | SLA vs.Φarea | |||||||||||||
| Constant (a) | Slope (b) | P value | r2 | Constant (a) | Slope (b) | P value | r2 | Constant (a) | Slope (b) | P value | r2 | ||||||
| Trema | SLPs | −1·91 | 1·01 | <0·001 | 0·98 | −8·81 | 0·57 | 0·002 | 0·48 | 4·39 | −1·38 | 0·13 | 0·16 | ||||
| Ochroma | SLPs | −2·47 | 1·11 | <0·001 | 0·92 | −8·32 | 0·49 | <0·001 | 0·63 | 4·82 | −0·03 | 0·73 | 0·01 | ||||
| Cecropia | SLPs | −2·46 | 1·05 | <0·001 | 0·89 | −8·33 | 0·51 | 0·001 | 0·52 | 5·18 | 0·23 | 0·73 | 0·01 | ||||
| Couratari | LLPs | 2·54 | 1·12 | <0·001 | 0·91 | −8·37 | 0·44 | 0·002 | 0·49 | 4·90 | 0·02 | 0·80 | 0·004 | ||||
| Rinereocarpus | LLPs | −2·38 | 1·15 | <0·001 | 0·85 | −8·77 | 0·45 | 0·01 | 0·46 | 4·76 | −0·10 | 0·24 | 0·10 | ||||
| Brosimum | LLPs | −1·93 | 0·90 | <0·001 | 0·96 | −6·98 | 0·10 | 0·30 | 0·07 | 3·41 | −0·24 | 0·16 | 0·11 | ||||
| Pseudolmedia | LLPs | −2·08 | 0·88 | <0·001 | 0·91 | −6·85 | 0·05 | 0·02 | 0·69 | 5·40 | 0·04 | 0·83 | 0·01 | ||||
| Bignoniaceae sp. | L | −2·31 | 0·99 | <0·001 | 0·84 | −8·01 | 0·35 | 0·21 | 0·04 | 4·84 | 0·01 | 0·96 | 0·001 | ||||
| Hippocrateaceae sp. | L | −1·69 | 0·90 | <0·001 | 0·93 | −6·55 | 0·08 | 0·63 | 0·02 | 3·83 | −0·16 | 0·39 | 0·05 | ||||
| Uncaria | L | 2·03 | 0·96 | <0·001 | 0·98 | −8·06 | 0·35 | 0·001 | 0·57 | 2·23 | −0·43 | 0·001 | 0·56 | ||||
P values of the regression analysis are shown. r2 denotes correlation coefficient.
SLPs, LLPs and L denote short-lived pioneers, long-lived pioneers and lianas, respectively.
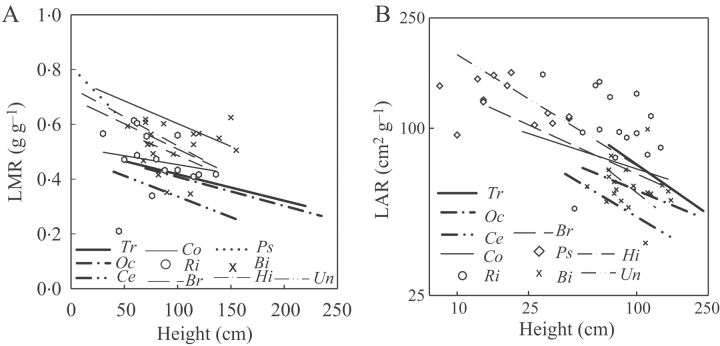
LMR and LAR values decreased with plant height within species (Fig. 3A and B). Species did not differ in the slopes of the relationships between LMR and height and LAR and height but they differed in intercepts. LMR values for a given height were lower for SLPs than the other species. LAR values for a given height were also lower for SLPs but the differences with the other species were not significant (Table 3) (Sidak at P<0·05). Regressions per species of LMR and LAR with plant height were significant for 2 and 3 out of the ten species, respectively (Table 4). All three SLPs had approx. 2-fold lower LMR values than the LLPs and lianas when compared at the same height. LMR values were also 2-fold different between the LLPs. The mean specific leaf area of plants (SLA) exhibited a negative correlation with the mean light intensity per unit of leaf area (Φarea). However, at the species level SLA and Φarea were only significantly correlated in Uncaria (Table 4).
Fig. 3.
Regression lines per species of (A) LMR (leaf mass ratio) vs. plant height and (B) LAR (leaf area ratio) vs. plant height. Lines denote significant regression at P<0·05. Symbols denote species with no significant correlation. For an explanation of the abbreviations, see Fig. 2.
Most of the species held most of their leaves horizontally oriented (0–30°) (data not shown) (ANOVA F(9,138)=2·68 P=0·007). Only Ochroma displayed half of its leaves at angles between the 30° and 60°.
Light (PPFD) interception
The species differed in the absolute daily amount of PPFD captured per individual plant (Φd) and per unit leaf area (Φarea, Table 2) with these values being higher for the two tallest species (Trema and Ochroma) than for the others. Φd and above-ground mass were highly correlated (Table 4). Species did not differ in the slopes of the relationships between Φd and plant mass, but they differed in the intercept with an SLP (Trema) and an LLP (Rinereocarpus) intercepting more light for a given mass than the other species (Table 4).
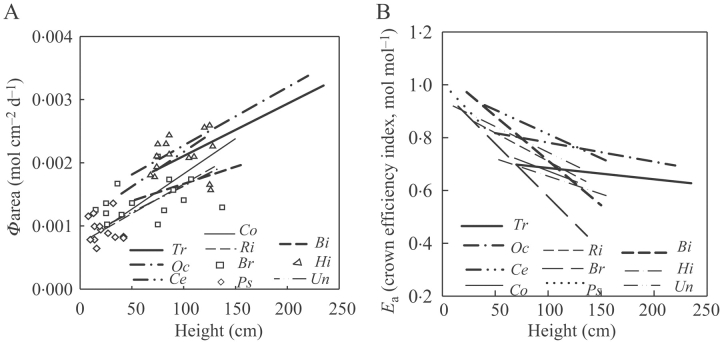
The Φarea increased with height (Fig. 4A). Species differed in the slopes of the relationship between Φarea and height with shorter individuals of SLPs having higher Φarea than the shorter individuals of the LLPs and lianas with the exception of Hippocrateacea species and Bignoniaceae species. Species differed in the slopes of the relationship Φarea, and in Ea and height (Table 3). Ea decreased with height (Fig. 4C), but much less so in the SLPs than in the other species. Consequently among plants >0·5 m tall the SLPs had the highest Ea values.
Fig. 4.
Regression lines per species of (A) Φarea (PPFD per leaf area per day) vs. plant height and (B) Ea (crown efficiency index) vs. plant height. Lines denote significant regression at P<0·05. Symbols denote species with no significant correlation. For an explanation of the abbreviations, see Fig. 2.
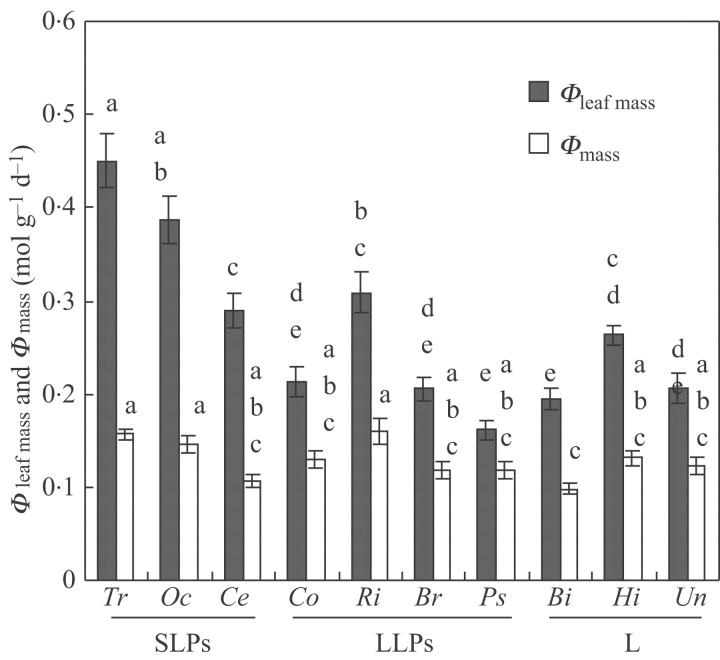
The species differed with respect to PPFD per leaf mass Φleaf mass and Φmass. The SLPs Trema and Ochroma attained the highest Φleaf mass values of all species (ANOVA P<0·005, Table 2). Pair-wise comparisons showed no differences in Φmass between the SLPs and LLPs and liana species (Fig. 5).
Fig. 5.
Mean instantaneous Φleaf mass (PPFD per leaf mass per day; filled columns) and Φmass (PPFD per above-ground mass per day; open columns) of species. For an explanation of the abbreviations, see Fig. 2. Bars denote standard errors. Similar letters denote no significant differences between species at P<0·05. SLPs, LLPs and L denote short-lived pioneers, long-lived pioneers and lianas, respectively.
DISCUSSION
The prediction that SLPs possess morphological traits that facilitate a high light interception per unit mass was not clearly supported by the present data. Per day, the SLPs Trema and Ochroma intercepted more light per unit leaf mass (Φleaf mass), but not in terms of (Φmass), than the other species. The LLPs and lianas intercepted a similar amount of light per unit of total above-ground mass (Φmass) than SLPs.
High Φleafmass and Φmass can result from being tall or by having an efficient crown structure (Ea). In this way, plants can position their leaves favourably relative to the light climate in the canopy realizing a high light interception per unit leaf area (Φarea) (Valladares et al., 2002). In the present study differences in Φarea between the tallest SLPs (Trema and Ochroma) and the other species lower in the canopy were significant but small. This result contrasts with those reported for herbaceous stands (2- to 5-fold) (Hirose and Werger, 1995; Anten and Hirose, 1999; Werger et al., 2002). This is probably because in the very young stand studied here, height differences were still small and the LAI was low (1·6) so that a relatively large amount of light penetrated deep into the canopy (Fig. 1).
Tall stature can be achieved through an efficient conversion of biomass to height growth by producing thin stems made of low density wood. With the exception of Trema, the SLPs, however, did not achieve a greater height for a given mass than trees from the other groups. Trema plants also had the most slender stems for their height of all species in the study. Slender stems facilitate rapid height growth but also imply reduced mechanical stability (Putz et al., 1983; Niklas, 1992; King, 1994; Montgomery and Chazdon, 2001). It is possible that the latter issue is not a major problem for Trema because, in dense vegetation, trees of this species have short life spans, and therefore do not need to invest as much in mechanical stability as the longer-lived Ochroma and Cecropia. Observations in the study area showed that Trema was not common in plots >4 years old, whereas Ochroma and Cecropia were found even after 25–30 years of succession.
The SLPs had higher crown efficiencies for light capture (Ea) than the other species, at least when comparing among individuals >0·5 m tall. As leaf inclination angles did not differ significantly between the groups, this greater Ea was mainly due to the fact the SLPs had shallower crowns with leaves being concentrated towards the top of the plant where they are favourably positioned relative to the light climate. These differences in Ea between SLPs and the other species probably reflect their different light requirements (Valladares et al., 2002). The SLPs, being shade intolerant, need to continuously produce new leaves at the top of the canopy to prevent being shaded by neighbours. Older leaves are probably dropped to provide resources for new leaf production and because, once they are shaded, their net photosynthetic contribution to the plant is small. By contrast, this continuous redeployment of resources is a cost that shade-tolerant plants cannot afford (King, 1994; Poorter and Werger, 1999; Valladares et al., 2002). Keeping this argument, it was observed that the leaf life spans of SLPs were considerably shorter than those of other species.
Apart from maintaining a high Φarea, efficient acquisition of light can also be achieved through a high allocation of mass to leaves and the formation of thin leaves with a high SLA, leading to a large leaf area per unit of plant mass (LAR; Hirose and Werger, 1995). Previous studies report that pioneers have a higher leaf mass ratio (LMR) than shade-tolerant species, especially under shaded conditions (see Poorter, 2006). In contrast to these studies, SLPs were found to have much lower mean LMR values than the other tree groups also when LMR was compared between plants of the same height (see also Sterck et al., 2001). This discrepancy in the LMR data is probably related to the fact that previous studies (Poorter, 1999, 2006) were conducted with plants grown in a garden experiment at relatively low density while the present study was conducted on plants growing in dense natural vegetation. Plants typically respond to the close proximity of neighbours by increasing mass investment to height growth (Smith, 1982; Anten and Hirose, 1998; Poorter, 1998), but the magnitude of this response can differ considerably between species. Among both temperate and tropical forest trees early successional species have been observed to exhibit greater responses to crowding than late successional ones (Kitajima, 1994; Gilbert et al., 2001).
Taller stature is usually associated with a lower leaf allocation because, in order to maintain mechanical stability, taller plants have to invest disproportionate amounts of mass in stems for support (Niklas, 1992; Anten and Hirose, 1998). However, a low LMR can also be the result of low leaf longevity (King, 1994; Claveau et al., 2005). As noted above, the progressive production of leaves at the top of the canopy by SLPs and associated redeployment of resources can result in reduced leaf longevity.
If the SLPs did not have higher efficiency of biomass use for light capture, are there other characteristics that may contribute to their early competitive advantage? Garden experiments have shown that under relatively high light conditions, the differences in growth rates of pioneers relative to other successional categories is more closely associated with growth per unit of leaf area (NAR) than with LAR (Poorter, 1999). The former, in turn, is strongly determined by physiological traits such as leaf photosynthetic capacity (Pmax). It has indeed been shown that the SLPs have higher Pmax values than later successional species (Ellsworth and Reich, 1996). A study on grasslands (Anten and Hirose, 2003) revealed that the competitive advantages of certain species in dense vegetation are associated not only with morphological traits that facilitate a high Φmass, like those studied here, but also with physiological ones that enable plants to efficiently use absorbed light for growth.
It should be noted that the present analysis is based on above-ground mass and that interspecific differences in root mass fractions were not accounted for. This difference may not be severe given that studies of early pioneers and intermediate species at medium and high irradiance levels showed that the two groups do not differ in root mass fraction (Veneklaas and Poorter, 1998).
The hypothesis that lianas, owing to the fact they (will eventually) climb, need to allocate less biomass for support and can therefore use more resources to produce leaves or for additional height growth that results in a higher Φmass was not supported by the present data. Φmass and LMR values of lianas were not different from those of the tree species. It was also found that self-supporting, young lianas had stem densities that were similar to similarly sized tree seedlings. It was further reported that the transition from the self-supporting to climbing growth is associated with substantial changes in wood anatomy (Gallenmüller et al., 2004) and other wood properties (J. Putz, University of Gainsville, Florida, USA, pers. comm.). Hence, it is possible that because lianas go through a self-supporting seedling phase, very early in succession they are not very different from trees in terms of mass allocation and efficiency of light capture, but may become more efficient once they have started climbing.
In conclusion, during the first year of succession, crown structure and morphological characteristics of the SLPs did not result in a greater efficiency of biomass use for light capture (Φmass) compared with the LLPs nor were the lianas more efficient than the trees. While the SLPs were taller and had shallower crowns with less self-shading resulting in higher crown display efficiencies (Ea), the LLPs and lianas exhibited larger leaf mass and area ratios. Thus, due to the trade-off between crown structure and biomass allocation SLPs, LLPs and lianas intercept similar amount of light per unit mass which may contribute to the ability of the latter two groups to persist.
ACKNOWLEDGEMENTS
We thank Rene Boot and Jack Putz for their valuable suggestions on the manuscript. We are also indebted to Ofelia La Fuente amd Katia Cuellar (Universidad Tećnica del Beni) and to Adhemar Saucedo, Luis Apaza, Nazareno Martinez, Rene Aramayo, Nico Divico, Miguel Cuadiay, Victor Aguada, Gabriel Ricaldi and Jorge Okita (PROMAB) for their assistance. Eduardo Chono kindly allowed us to use his plot.
LITERATURE CITED
- Anten NPR. Modelling canopy photosynthesis using parameters determined from simple non-destructive measurements. Ecological Research. 1997;12:77–88. [Google Scholar]
- Anten NPR. Optimal photosynthetic characteristics of plants in stands. Annals of Botany. 2005;95:495–506. doi: 10.1093/aob/mci048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anten NPR, Hirose T. Interspecific differences in aboveground growth patterns result in spatial and temporal partitioning of light among species in a tall-grass meadow. Journal of Ecology. 1999;87:583–597. [Google Scholar]
- Anten NPR, Hirose T. Biomass allocation and light partitioning among dominant and subordinate individuals in Xanthium canadense stands. Annals of Botany. 1998;82:665–673. [Google Scholar]
- Anten NPR, Hirose T. Shoot structure, leaf physiology and daily carbon gain of species in a tall-grass meadow. Ecology. 2003;84:955–968. [Google Scholar]
- Bongers F, Popma J. Leaf characteristics of the tropical rain forest flora of Los-Tuxtlas, Mexico. Botanical Gazette. 1990;151:354–365. [Google Scholar]
- Caballe G. Le port autoportant des lianes tropicales: une synthèse des stratégies de croissance. Canadian Journal of Botany. 1998;76:1703–1716. [Google Scholar]
- Chazdon RL. Photosynthetic plasticity of two rain forest shrubs across natural gap transects. Oecologia. 1992;92:586–595. doi: 10.1007/BF00317853. [DOI] [PubMed] [Google Scholar]
- Claveau Y, Messier C, Comeau PG. Interacting influence of light and size on aboveground biomass distribution in sub-boreal conifer saplings with contrasting shade tolerance. Tree Physiology. 2005;25:373–384. doi: 10.1093/treephys/25.3.373. [DOI] [PubMed] [Google Scholar]
- Dalling JW, Winter K, Hubbell SP. Variation in growth responses of neotropical pioneers to simulated forest gaps. Functional Ecology. 2004;18:725–736. [Google Scholar]
- De Walt SJ, Schnitzer SA, Denslow JS. Density and diversity of lianas along a chronosequence in a central Panamanian lowland forest. Journal of Tropical Ecology. 2000;16:1–19. [Google Scholar]
- Depury DGG, Farquhar GD. Simple scaling of photosynthesis from leaves to canopies without the errors of big leaf models. Plant, Cell and Environment. 1997;20:537–557. [Google Scholar]
- Ellsworth DS, Reich PB. Photosynthesis and leaf nitrogen in five Amazonian tree species during early secondary succession. Ecology. 1996;77:581–594. [Google Scholar]
- Falster DS, Westoby M. Leaf size and angle vary widely across species: what consequences for light interception? New Phytologist. 2003;158:509–525. doi: 10.1046/j.1469-8137.2003.00765.x. [DOI] [PubMed] [Google Scholar]
- Finegan B. Pattern and process in neotropical secondary rainforest: the first 100 years of succession. TREE. 1996;11:119–124. doi: 10.1016/0169-5347(96)81090-1. [DOI] [PubMed] [Google Scholar]
- Gallenmüller F, Rowe N, Speck T. Development and growth from of the neotropical liana Croton nuntians: the effect of light and mode of attachment on the biomechanics of the stem. Journal of Plant Growth Regulation. 2004;23:83–97. [Google Scholar]
- Gates DM. Biophysical ecology. Berlin: Springer; 1980. [Google Scholar]
- Gerwing JJ. Life history diversity among six species of canopy lianas in an old growth forest of the eastern Brazilian Amazon. Forest Ecology and Management. 2004;190:57–72. [Google Scholar]
- Gilbert IR, Jarvis PG, Smith H. Proximity signal and shade avoidance differences between early and late successional trees. Nature. 2001;411:792–795. doi: 10.1038/35081062. [DOI] [PubMed] [Google Scholar]
- Goudriaan J. Crop micrometeorology: a simulation study. Wageningen: Pudoc; 1977. Simulation Monographs. [Google Scholar]
- Goudriaan J. The bare bones of leaf angle distribution in radiation models for canopy photosynthesis and energy exchange. Agricultural and Forest Meteorology. 1988;43:155–169. [Google Scholar]
- Hirose T. Development of the Monsi-Saeki theory on canopy structure and function. Annals of Botany. 2005;95:483–494. doi: 10.1093/aob/mci047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose T, Werger MJA. Canopy structure and photon flux partitioning among species in a herbaceous plant community. Ecology. 1995;76:466–474. [Google Scholar]
- Horn HS. The adaptive geometry of trees. Princeton, NJ: Princeton University Press; 1971. [Google Scholar]
- King DA. Allometry of saplings in understorey trees of a Panamaniam forest. Functional Ecology. 1990;4:27–32. [Google Scholar]
- King DA. Influence of light level on the growth and morphology of saplings in a Panamanian forest. American Journal of Botany. 1994;81:948–957. [Google Scholar]
- Kitajima K. Relative importance of photosynthetic traits and allocation patterns as correlates of seedling shade tolerance of 13 tropical trees. Oecologia. 1994;98:419–428. doi: 10.1007/BF00324232. [DOI] [PubMed] [Google Scholar]
- Kitajima K, Mulkey SS, Wright SJ. Variation in crown light utilization characteristics among tropical canopy trees. Annals of Botany. 2005;95:535–547. doi: 10.1093/aob/mci051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohyama T. Significance of the architecture and allometry in saplings. Functional Ecology. 1987;1:399–404. [Google Scholar]
- Montgomery RA, Chazdon RL. Forest structure, canopy architecture, and light transmittance in tropical wet forests. Ecology. 2001;82:2207–2718. [Google Scholar]
- Niklas KJ. Plant biomechanics. Chicago, IL: University of Chicago Press; 1992. [Google Scholar]
- Peña-Claros M. Secondary forest succession: processes affecting the regeneration of Bolivian tree species. Utrecht University; 2001. (PROMAB Scientific Series 3). Forest successional stage affects survival and growth of rain forest tree species differing in shade tolerance. PhD Thesis, PROMAB Riberalta. [Google Scholar]
- Peña-Claros M. Changes in forest structure and species composition during secondary succession in the Bolivian Amazon. Biotropica. 2003;35:450–461. [Google Scholar]
- Pompa J, Bongers F. The effect of canopy gaps on growth and morphology of seedlings of rain forest species. Oecologia. 1988;75:623–632. doi: 10.1007/BF00776429. [DOI] [PubMed] [Google Scholar]
- Poorter L. Competition among rain forest tree seedlings during gap phase regeneration. Seedling growth of Bolivian rain forest tree species in relation to light and water availability. PROMAB-Utrecht University; 1998. PhD Thesis. [Google Scholar]
- Poorter L. Growth responses of fifteen rain forest tree species to a light gradient: the relative importance of morphological and physiological traits. Functional Ecology. 1999;13:396–410. [Google Scholar]
- Poorter L. Resource capture and use by tropical forest tree seedlings and its consequences for competition. In: Burslem DFRP, Pinard M, Hartley S, editors. Biotic interactions in the tropics. Cambridge: Cambridge University Press; 2006. (in press) [Google Scholar]
- Poorter L, Werger MJA. Light environment, sapling architecture, and leaf display in six rain forest tree species. American Journal of Botany. 1999;86:1464–1473. [PubMed] [Google Scholar]
- Poorter L, Bongers L, Bongers F. Architecture of 54 moist-forest tree species: traits, trade-offs, and functional groups. Ecology. 2006;87:1289–1301. doi: 10.1890/0012-9658(2006)87[1289:aomtst]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Putz FE. The natural history of lianas in Barro Colorado Island, Panama. Ecology. 1984;65:1713–1724. [Google Scholar]
- Putz FE. Vine ecology. University of Florida Gainsville, FL: Department of Botany; 2006. Online review. http://www.ecology.info/vines.htm . [Google Scholar]
- Putz FE, Coley PD, Lu K, Montalvo A, Aiello A. Uprooting and snapping of trees: structural determinants and ecological consequences. Canadian Journal of Forest Research. 1983;13:1011–1020. [Google Scholar]
- Smith H. Light quality, photoperception and plant strategy. Annual Review of Plant Physiology. 1982;12:481–518. [Google Scholar]
- Schnitzer SA, Bongers F. The ecology of lianas and their role in forests. Trends in Ecology and Evolution. 2002;17:223–230. [Google Scholar]
- Sterck JF, Bongers F, Newbery DM. Tree architecture in a Bornean lowland rain forest: intraspecific and interspecific patterns. Plant Ecology. 2001;153:279–292. [Google Scholar]
- Valladares F, Wright SJ, Lasso E, Kitajima K, Pearcy RW. Plastic phenotypic response to light of 16 congeneric shrubs from a Panamanian rainforest. Ecology. 2000;81:1925–1936. [Google Scholar]
- Valladares F, Skillman JB, Pearcy RW. Convergence in light capture efficiencies among tropical forest understory plants with contrasting crown architectures: a case of morphological compensation. American Journal of Botany. 2002;89:1275–1284. doi: 10.3732/ajb.89.8.1275. [DOI] [PubMed] [Google Scholar]
- Veneklaas EJ, Poorter L. Growth and carbon partitioning of tropical tree seedlings in contrasting light environments. In: Lambers H, Poorter H, van Vuuren MMI, editors. Variation in plant growth. Leiden: Backhuys Publishers; 1998. pp. 337–361. [Google Scholar]
- Werger MJA, Hirose T, During H, Heil GW, Hikosaka K, Ito T, et al. Light partitioning among species and species replacement in early successional grasslands. Journal of Vegetation Sciences. 2002;13:615–626. [Google Scholar]
- Whitmore TC. Canopy gaps and the two major groups of forest tree. Ecology. 1989;70:536–538. [Google Scholar]