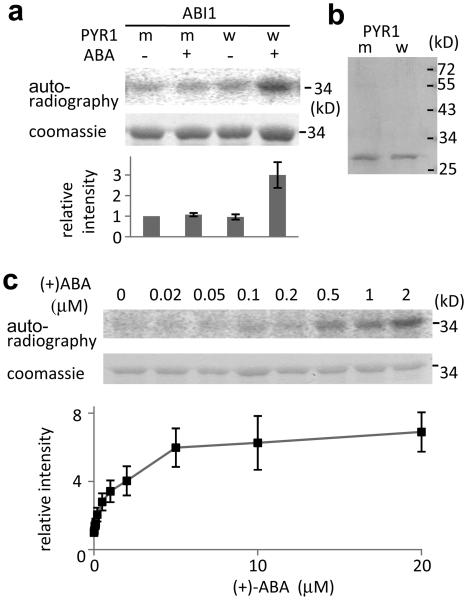
Figure 3. The combined effect of ABA, PYR1 and ABI1 on SnRK2.6 phosphorylation of GST-ABF2 fragment in vitro.
a, Reconstitution of ABA regulation of ABF2 phosphorylation. MBP-SnRK2.6 treated with GST-ABI1 and His-tagged wild type PYR1 (w) or mutated PYR1P88S (m) in the absence (−) or presence (+) of 2 μM (+)-ABA was incubated with GST-ABF2 fragment (amino acids Gly73 to Gln119) in the presence of [γ32P]-ATP. Coomassie staining, autoradiography and relative radioactivities of GST-ABF2 fragment are shown. Radioactivities of GST-ABF2 fragment were normalized, taking the radioactivity of the band with PYR1P88S in the absence of ABA as 1 (mean ± s.e.m., n = 5). b, Coomassie staining of PYR1 (w) and PYR1P88S (m). c, ABA dose response. MBP-SnRK2.6, GST-ABI1 and His-PYR1 were incubated with different concentrations of (+)-ABA before the kinase assay using GST-ABF2 fragment as substrate. Coomassie staining, autoradiography and relative radioactivities (taking the radioactivity of the band in the absence of ABA as 1; mean ± s.e.m., n = 9 for < 5 μM, n = 4 for 5 μM or more) of GST-ABF2 fragment are shown.