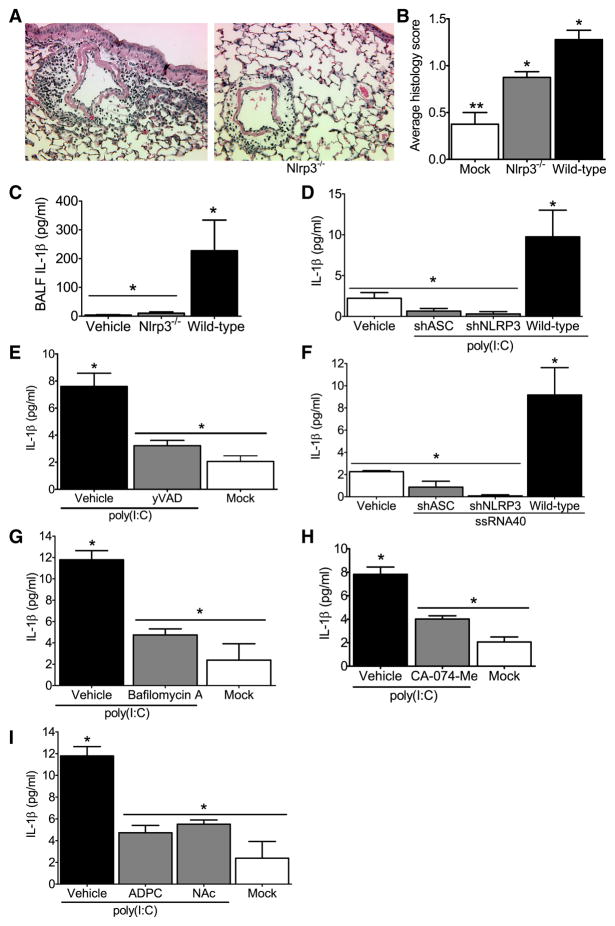
Figure 6. The NLRP3 inflammasome is required for airway inflammation induced by nucleic acid analogs.
Wild type and Nlrp3−/− mice received either 2 doses (50μg/dose) of poly(I:C) or vehicle on alternating days and were harvested 24hrs after the second dose was administered. (A–B) Poly(I:C) challenged animals demonstrated increased airway inflammation but less inflammatory cell influx was observed in Nlrp3−/− mice. (C) Histology scoring confirmed a significant attenuation in airway inflammation in the Nlrp3−/− mice (*p<0.05). (D) Significant decreases were observed in BALF IL-1β in the Nlrp3−/− mice compared to the wild type animals (*p<0.05). Vehicle challenged wild type (n=4); wild type, n=8; Nlrp3−/−, n=8. (E–G) IL-1β induction by viral RNA analogs in human monocytic cells is mediated by NLRP3 and ASC. (E) IL-1β secretion stimulated by the viral dsRNA analog poly(I:C) was significantly reduced in the shNLRP3 and shASC knockdown THP-1 cell lines and (F) following treatment with the caspase-1 specific inhibitor yVAD-CHO (*p<0.05). (G) IL-1β secretion stimulated by ssRNA40 was also significantly reduced in the shNLRP3 and shASC containing cells (*p<0.05). All knockdown studies are representative of at least 3 independent experiments. (H–J) Poly(I:C) mediated IL-1β maturation requires lysosomal maturation and ROS production in human monocytes. (H) The lysosome inhibitor bafilomycin A (100nM), (I) the cathepsin B inhibitor CA-074-Me (50μM), and (J) the ROS inhibitors APDC (100μM) and NAc (50μM) all significantly attenuated IL-1β production (*p<0.05).