Abstract
It has been reported that Ocimum sanctum L. (OS) leaves decrease serum lipid profile in normal and diabetic animals. No experimental evidences support the anti-hyperlipidemic and antioxidative actions against hypercholesterolemia. Moreover the identity of the specific chemical ingredients in OS leaves responsible for these pharmacological effects are unknown. Since OS leaves are rich in essential oil (EO). Therefore the present study was conducted to investigate the anti-hyperlipidemic and antioxidative activities of EO extracted from OS leaves in rats fed with high cholesterol (HC) diet. EO was extracted by the hydrodistillation method and the chemical constituents were then identified by Gas Chromatography-Mass Spectrometry. The experiment was performed in Male Wistar rats fed with 2.5 g%(w/w) of cholesterol diet for seven weeks. During the last 3 weeks, rats were daily fed with EO. The results showed that phenyl propanoid compounds including eugenol and methyl eugenol were the major constituents of EO. EO suppressed the high serum lipid profile and atherogenic index as well as serum lactate dehydrogenase and creatine kinase MB subunit without significant effect on high serum levels of aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase in rats fed with HC diet. In addition, EO was found to decrease the high levels of thiobarbituric acid reactive substances (TBARS), glutathione peroxidase (GPx) and superoxide dismutase (SOD) without impacting catalase (CAT) in the cardiac tissue while in the liver, it decreased high level of TBARS without significantly effecting GPx, SOD and CAT. Histopathological results confirmed that EO preserved the myocardial tissue. It can be concluded that EO extracted from OS leaves has lipid-lowering and antioxidative effects that protect the heart against hypercholesterolemia. Eugenol that is contained in EO likely contribute to these pharmacological effects.
Keywords: high fat diet, Ocimum sanctum L., antioxidant, liver function, cardiac function
Introduction
Hypercholesterolemia is widely known to be the major risk factor for the development of cardiovascular diseases [1, 2]. The modern lifestyle with high fat diet and less physical activity significantly contribute to hypercholesterolemia and cardiovascular diseases [1, 3]. Oxidative stress induced by reactive oxygen species (ROS) also plays an important role in the etiology of several diseases including atherosclerosis and coronary heart disease [4, 5]. Hypercholesterolemia has been found to induce oxidative stress in various organs such as the liver, heart and kidney [6]. It has been shown that majority of the plants with antioxidant products have a potential role in protecting people from several illnesses such as cardiovascular diseases [5, 7]. There are several kinds of medicinal plants with hypolipidemic and antioxidative activities. One of those plants, Ocimum sanctum L. (OS), commonly used as a vegetable, has shown its potential to be therapeutic in averting several diseases in various Asian countries including India and Thailand. OS is a small herb, native to tropical and subtropical regions. It is known as Tulsi or Holy Basil in English and India. It has been shown that 2% of dried OS leaf powder supplemented in the diet can lower serum lipid profile and partially protect the liver in diabetic rats [8]. It has also been shown that OS leaf extracts can protect the liver from heavy metals [9] and prevent isoproterenal-induced myocardial necrosis in rats [10]. Even though OS leaves have hypolidemic and organ protective effects against various stress conditions, yet there are no research studies providing evidences for its anti-hyperlipidemic and antioxidative effects to protect the primary risk organs against hypercholesterolemia. Moreover, the chemical constituents in OS leaves contributing to these actions have not yet been identified. It is known that OS leaves are riched in essential oils (EO), and the primary risk organs of hypercholesterolemia are the liver and heart. Therefore, the present study was conducted to investigate anti-hyperlipidemic and antioxidative actions of EO extracted from OS leaves to protect the liver and heart in rats fed with high cholesterol diet. The chemical composition of EO was also identified.
Materials and Methods
Extraction of essential oils from Ocimum sanctum L. leaves
OS fresh leaves were obtained from the Institute of Thai Traditional Medicine, the Ministry of Public Health of Thailand. Fresh leaves of OS were washed in tap water and then cut into small pieces. The EO from OS leaves was extracted by the hydrodistillation method as described by the Association of Official Analytical Chemists (method 962.17, AOAC, 1990). After the extraction process, the percent yield of EO was 1.82 ml/100 g of fresh OS leaves. The EO was collected and stored at 4°C before analyzing its chemical constituents by Gas Chromatography-Mass Spectrometry (GC-MS).
Identification of volatile constituents using GC-MS
The EO was diluted to 1:100 in methanol before being injected into the GC-MS system. The Varian Saturn III instrument was used for Gas Chromatography-Mass Spectrometry analysis. The column was a DB-5 fused silica capillary column 30 m (0.25 mm i.d., 0.25 µM film thickness). Oven temperature programming was 50–240°C with an increase of 3°C/min. Injector and detector temperatures were 240°C. The volume of the injector was 1 µl; the split ratio was 100:1, the accelerating voltage was 1700 volts, and the carrier gas was helium. Identification was based on sample retention time data and comparison with authentic standards, electron impact-mass spectra (EI-MS) data and computer matching using NIST library. (NIST = NIST. Mass Spectral Library, The National Institute of Standards and Technology, UK, 1998).
Animal preparation
Male Wistar rats weighing between 90–120 g were purchased from the Animal Center, Salaya Campus, Mahidol University, Thailand. The rats were cared for in accordance with the principles and guidelines of the Institutional Animal Ethics Committee of Rangsit University, which is under The National Council of Thailand for Animal Care. The rats were housed in a 12-h light–dark cycle room with controlled temperature at 25 ± 2°C and fed with standard rat food and tap water ad libitum. Hypercholesterolemia was induced by adding 2.5 g% (w/w) cholesterol powder and 6% (v/w) palm oil to the food. Three groups of seven rats each were fed on the following diet for 7 weeks: group I, normal diet for a control; group II, a high cholesterol (HC) diet; and group III, a HC diet and EO was intragastrically administered daily for the last three weeks.
From our previous study, supplementation of 2% dried OS leaf powder in diet for three weeks showed lipid-lowering effect and partially protected the liver in diabetic rats [8]. The percent yield of EO was 1.82 ml/100 g of fresh OS leaves which was equivalent to 1.82 ml/10.13 g dried OS leaf powder. The average amount of dried OS leaf powder consumption by each rat was 4.45 g/kgbw/day. Therefore the daily dose of EO administered in this study was calculated based on these values and was approximately 80 µl/kgbw/day. EO was dissolved in liquid paraffin emulsion at the concentration of 30 µl/ml. During the last three weeks of the experiment, 0.8 ml of liquid paraffin emulsion was daily fed to group I and II whereas group III was daily fed with EO. To improve absorption, food was withheld for two hours before EO or liquid paraffin emulsion was administered. Body weight and food consumption were recorded weekly throughout seven weeks.
At the end of the study, the rats were fasted overnight and were anesthetized by intraperitoneal injection with zolitil (40 mg/kgbw) plus xylazine (3 mg/kgbw). Blood was collected from the abdominal vein to determine the serum lipid profile: total cholesterol, triglyceride, low density lipoprotein cholesterol (LDL-C), and high density lipoprotein cholesterol (HDL-C). The atherogenic index (AI) was later calculated as the ratio of TC-HDL-C/HDL-C.
Evaluation of liver and cardiac function
Liver function was evaluated by assessing for serum alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (AP) levels. Cardiac function was also assessed by measuring the serum lactate dehydrogenase (LDH) and creatine kinase MB subunit (CK–MB) levels.
Assessment of lipid peroxide and the activity of antioxidant enzymes in both the liver and heart
At the end of the study, rat was anesthetized and the jugular vein was canulated to perfuse ice cold normal saline to remove red blood cells. Immediately after perfusion, both the liver and heart were isolated, cleaned and weighed. Both organs were kept at −80°C until further analysis was done.
Assessment of tissue lipid peroxide content
Lipid peroxides in both liver and heart were assessed with thiobarbituric acid reactive substances (TBARS) as described previously [11]. TBARS was expressed in nmole of malondialdehyde (MDA)/mg protein using 1,1,3,3-tetraethoxy propane as a standard. Tissue protein levels were determined with the Lowry’s method [12].
Assessment of the activity of tissue antioxidant enzymes
Antioxidant enzymes such as glutathione peroxidase (GPx), catalase (CAT) and superoxide dismutase (SOD) were determined in the liver and heart. Liver and cardiac tissue homogenates were prepared by homogenizing the tissues in a 0.1 M phosphate buffer pH 7.4. The homogenate was then centrifuged at 3,000 rpm, 4°C for 10 min. Supernatant was collected and centrifuged again at 7,800 g, 4°C for 30 min. The supernatant fraction was collected and further centrifuged at 136,000 g, 4°C for 60 min. The final supernatant was then analyzed for estimation of GPx, CAT and SOD using the procedures described by Tapple [13], Luck [14] and Winterbourn et al. [15] respectively.
Evaluation of the liver and cardiac morphology
After the liver and heart were isolated, cleaned and dried, then fixed in a buffer solution of 10% neutral buffered formalin. For histopathological observations, longitudinal sections of myocardial tissue were cut at the macroscopic lesions. As for the liver, sections were cut through the macroscopic lesions including capsules. The sections were further cut to 5 microns thickness and stained with haematoxylin and eosin (H&E) [16].
Biochemical assay
Serum total cholesterol, triglyceride and HDL-C were assayed by using an enzymatic kit (Gesellschaft Für Biochemica und Diagnostica GmbH, Germany). LDL-C was calculated by using the equation: LDL-C = TC–(HDL-C)–(triglyceride/5). Serums levels for AST, ALT, AP, LDH and CK–MB were measured by using an enzymatic kit (Randox Laboratories, UK).
Statistical analysis
All values are presented as mean ± SEM. The results were analyzed by ANOVA. The Ducan multiple rank test was performed to determine statistical significance among groups by using SPSS software version 11.5. Significant difference was accepted at the p<0.05.
Results
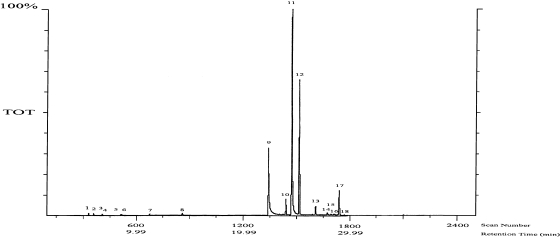
Fig. 1 shows GC of the EO extracted from OS leaves. The chemical constituents of EO are displayed in Table 1. The EO is predominantly composed mainly of phenylpropanoids (65.31%) eugenol and methyl eugenol. 9-epi (E)-Caryophyllene which is sesquiterpene compound, was found 23.68% in EO. Small amounts of monoterpene and oxygenated monoterpene were also found in EO.
Fig. 1.
Chromatogram of the essential oils extracted from OS leaves
Table 1.
Chemical composition of essential oils extracted from Ocimum sanctum L. leaves by hydrodistillation method
| Number of peak | Chemical compounds and structures | Retention time (min) | % Area | Molecular formula | Molar mass (g/mol) |
|---|---|---|---|---|---|
| mm | Monoterpene | ||||
| 1 | α-thujene | 5.48 | 0.19 | C10 H16 | 136.23 |
| 2 | camphene | 5.62 | 0.21 | C10 H16 | 136.24 |
| 3 | sabinene | 6.33 | t | C10 H16 | 136.23 |
| 4 | β-pinene | 6.39 | 0.13 | C10 H16 | 136.24 |
| 5 | limonene | 7.96 | 0.08 | C10 H16 | 136.24 |
| Oxygenated monoterpene | |||||
| 6 | 1,8-cineole | 8.11 | 0.06 | C10 H18 O | 154.249 |
| 7 | linalool | 10.59 | 0.3 | C10 H18 O | 154.25 |
| 8 | borneol | 13.59 | 0.54 | C10 H18 O | 154.249 |
| Sesquiterpene | |||||
| 10 | β-elemene | 23.07 | 2.64 | C15 H24 | 204.35 |
| 12 | 9-epi (E)-caryophyllene | 25.41 | 23.68 | C15 H24 | 204.36 |
| 13 | α-humulene | 25.8 | 1.5 | C15 H24 | 204.36 |
| 14 | γ-muurolene | 26.39 | 0.4 | C15 H24 | 204.35 |
| 15 | β-selinene | 26.78 | 0.11 | C15 H24 | 204.35 |
| 16 | α-selinene | 27.16 | 0.11 | C15 H24 | 204.35 |
| 17 | α-bulnesene | 27.48 | 4.6 | C15 H24 | 204.35 |
| 18 | δ-cadinene | 29.01 | 0.1 | C15 H24 | 204.35 |
| Phenyl propanoid | |||||
| 9 | eugenol | 21.26 | 18.25 | C10 H12 O2 | 164.2 |
| 11 | methyl eugenol | 23.61 | 47.06 | C11 H14 O2 | 178.23 |
t = trace
Body weight gain and food consumption were not significantly different in all groups of rats (data was not shown). Seven weeks of HC diet feeding raised serum total cholesterol, triglyceride, LDL-C and AI whereas HDL-C was slightly decreased (Table 2). EO treatment attenuated the high serum levels of cholesterol, LDL-C, AI, and normalized triglyceride and HDL-C. The HC diet significantly raised serums ALT, AST, AP, LDH and CK-MB (Table 3). In HC rats treated with EO, CK-MB and LDH levels were returned to normal levels, although ALT, AST and AP levels remained elevated.
Table 2.
Changes of liver weight, heart weight and serum lipid profile in normal rats, HC rats and HC rats treated with essential oils of Ocimum sanctum L. leaves
| group |
|||
|---|---|---|---|
| Normal | HC | HC + EO | |
| Organ weight (g/kgbw) | |||
| Liver | 32.5 ± 0.6a | 62.2 ± 1.5b | 65.8 ± 1.5b |
| heart | 3.53 ± 0.1 | 3.33 ± 0.09 | 3.30 ± 0.08 |
| Serum lipid (mg/dl) | |||
| Total cholesterol | 38 ± 2a | 133 ± 5b | 112 ± 3c |
| Triglyceride | 25 ± 1a | 50 ± 7b | 26 ± 3a |
| HDL-C | 20 ± 0ab | 17 ± 1a | 22 ± 2b |
| LDL-C | 13 ± 1a | 105 ± 6b | 85 ± 2c |
| AI | 0.9 ± 0.1a | 6.9 ± 0.8b | 4.4 ± 0.4c |
Values are expressed as mean ± SEM of seven rats per group. Values with different superscripts in the same row are significantly different at p<0.05.
Table 3.
Changes of serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (AP), lactate dehydrogenase (LDH) and creatine kinase MB subunit (CK–MB) in normal rats, HC rats and HC rats treated with essential oils of Ocimum sanctum L. leaves
| group |
|||
|---|---|---|---|
| Normal | HC | HC + EO | |
| ALT (U/L) | 25 ± 1a | 118 ± 17b | 119 ± 36b |
| AST (U/L) | 67 ± 3a | 166 ± 14b | 193 ± 22b |
| AP (U/L) | 117 ± 8a | 204 ± 8b | 213 ± 11b |
| LDH (U/L) | 530 ± 78a | 825 ± 86b | 487 ± 51a |
| CK-MB(U/L) | 499 ± 56a | 685 ± 35b | 514 ± 40a |
Values are expressed as mean ± SEM of seven rats per group. Values with different superscripts in the same row are significantly different at p<0.05.
Levels of hepatic lipid peroxides increased whereas GPx, CAT and SOD activities were decreased in HC rats (Table 4). EO depressed high level of liver TBARS without significantly effecting GPx, CAT and SOD activities. For cardiac tissue, TBARS was significantly increased while the activities of GPx and SOD were reduced in HC rats. In contrast, the rats treated with EO showed a huge reduction in cardiac TBARS and an increase in GPx and SOD activities. The cardiac CAT activity in HC rats treated with EO was increased to the similar level of normal rat but the level was not statistically significant difference from HC rats.
Table 4.
Effect of essential oils extracted from Ocimum sanctum L. leaves on lipid peroxide and antioxidant enzymes activity in liver and heart in HC rats.
| group |
|||
|---|---|---|---|
| Normal | HC | HC + EO | |
| Liver | |||
| TBARS (nmole MDA/mg protein) | 0.88 ± 0.06a | 1.38 ± 0.04b | 0.67 ± 0.06c |
| GPx (µmole/min/mg protein) | 1.3 ± 0.06a | 0.97 ± 0.02b | 1.07 ± 0.09b |
| CAT (µmole/min/mg protein) | 360 ± 16.6a | 275 ± 18b | 242 ± 15b |
| SOD (unit/mg protein) | 103 ± 13.9a | 40.1 ± 3.29b | 47.1 ± 5.17b |
| Heart | |||
| TBARS (nmole MDA/mg protein) | 1.0 ± 0.05a | 1.83 ± 0.05b | 0.35 ± 0.03c |
| GPx (µmole /mg protein) | 0.28 ± 0.02a | 0.20 ± 0.02b | 0.27 ± 0.03a |
| CAT (µmole/min/mg protein) | 6.95 ± 0.38a | 5.82 ± 0.31a | 6.80 ± 0.49a |
| SOD (unit/mg protein) | 25.3 ± 2.1a | 14.3 ± 1.2b | 61.4 ± 5.4c |
Values are expressed as mean ± SEM of seven rats per group. TBARS, thiobarbituric acid reactive substances; GPx, glutathione peroxidase; CAT, catalase; SOD, superoxide dismutase. Values with different superscripts in the same row are significantly different at p<0.05.
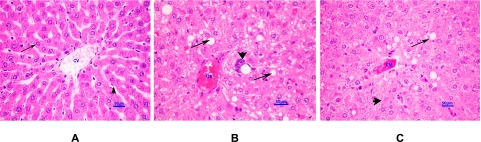
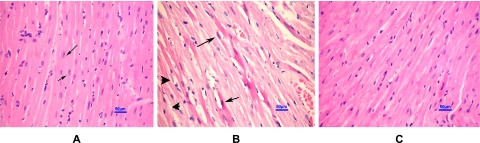
From the histopathological analysis, the normal hepatocyte had the round nucleus centrally and homogeneous cytoplasm (Fig. 2A). There are flat endothelial cells around central vein and sinusoid. The hepatic cord arrangement as a regular ray pattern was also seen in normal hepatocyte. The hepatocytes from HC rats showed diffused vacuolar degeneration and necrosis (loss of nucleus) which indicated that the cells were severely deteriorated (Fig. 2B). Endothelial lining of the central vein exhibited more cell injury with an increased accumulation of fat vacuoles in the hepatocytes. Hepatic cells did not recover or improve with EO usage even though there were fewer endothelium injuries and vacuole buildup (Fig. 2C). Myocardiocyte of normal rats had oval-elongate nucleus centrally and homogeneous cytoplasm (Fig. 3A). The HC rats exhibited a moderate dilation and thinning of the right ventricle wall with mild cardiac hypertrophy of the left ventricle. Furthermore, multi-focal vacuolar degeneration and necrosis or apoptosis were seen in the myocardial cells (Fig. 3B). In contrast, myocardial cell of HC rat treated with EO revealed normal general appearance (Fig. 3C).
Fig. 2.
Histopathological appearance of liver (H&E ×400). Normal hepatocyte had the round nucleus centrally (arrows), the flat endothelial cells (arrow-heads) are around the central vein (CV) (A). Diffuse vacuolar degeneration and necrosis of hepatocytes (arrows) and markedly focal fibrosis (arrow-head) were shown in HC rat (B). Hepatic cells of HC rat treated with EO (C), periacinar vacuolar cytoplasmic degeneration (arrows), 1–2 hepatocyte rows around central vein demonstrated hepatic cell degeneration and necrosis (arrow-head). Less injury of endothelium and less fat vacuole comparing to HC rat. HC, high cholesterol; EO, essential oil.
Fig. 3.
Histopathological appearance of myocardial cells (H&E ×400). Oval-elongate nucleus centrally and homogeneous cytoplasm (arrows) in normal myocardial cell (A). Multi-focal vacuolar degeneration and necrosis of myocardial cells (arrow-heads) in HC rat (B). Apoptosis of myocardiocytes were also observed (arrow in B). Normal general appearance of myocardial cells in HC rat treated with EO (C). HC, high cholesterol; EO, essential oil.
Discussion
Cardiovascular diseases, particularly coronary artery disease (CAD), have become a growing problem, and is the primary cause of mortality in both western countries and most parts of Asia [2, 17]. Several lines of evidences show that the improvement and incidence of CAD is associated with lowering hypercholesterolemia [1, 18]. To treat hypercholesterolemia, extensive interventions are recommended, including diet control, exercise and the use of hypocholesterolemic drugs. However some patients cannot tolerate the adverse effects from these drugs, such as liver damage [19] which indicates the need to find other safer yet efficacious alternatives. Many epidemiological studies have indicated that diet with high plant content can protect people from various serious diseases such as cancer, diabetes mellitus and cardiovascular disease [7, 20]. The present study shows that, as expected, seven weeks of HC diet feeding raised serum lipid profile and AI. EO treatment attenuated the high serum lipid profile and AI. This implies that EO contained in the OS leaves contributes to the anti-hyperlipidemic action of OS leaves, and could be effective to alleviate atherosclerosis which then eventually prevents the occurrence of CAD. The anti-hyperlipidemic activity of EO may be due to the suppression of liver lipid synthesis [21].
It is widely known that both the liver and heart are at risk in patients with hypercholesterolemia. Our results showed that HC diet markedly suppressed hepatic and cardiac functions as expressed by an augmentation of serum AST, ALT, AP, LDH and CK-MB (Table 3). Hepatic and cardiac lipid peroxidation as observed by the TBARS in both organs, were also increased in HC rats. Moreover, antioxidant enzyme activities in both organs were depressed. These results indicate that seven weeks of HC diet feeding induces oxidative stress to injure the liver and heart. Though OS leaves have been reported to protect both organs in various stress conditions as mentioned earlier, no experimental study was carried out to clarify its antioxidative action to protect both the liver and heart against hypercholesterolemia. EO treatment was able to normalize the high serum levels of LDH and CK-MB. Likewise, cardiac lipid peroxidation was markedly suppressed whereas the activity of cardiac antioxidant enzymes increased in rats treated with EO. This indicates that EO contained in the OS leaves had free radical scavenging activity which probably contributed to the cardioprotective effect against hypercholesterolemia.
As for the results from the liver, EO suppressed high levels of liver lipid peroxidation and slightly improved hepatocyte degeneration. However its effect was not powerful enough to protect the liver function as shown by the unchanged serum levels of ALT, AST and AP which remained high. This finding was quite difference from our previous study which showed that OS dried leaf powder supplemented in normal diet partially protected the liver in diabetic rats [8]. The reason may be that OS leaf powder contains several other kinds of chemical ingredients such as ursolic acid, apigenin, orientin, and linoleic acid. The hepatoprotective activity of OS leaves might be caused by other chemicals. The present study clearly shows that EO extracted from OS leaves may of the therapeutic importance not only as an anti-hyperlipidemic agent to prevent atherosclerosis but also as an cytoprotective agent to protect cardiac injury against hypercholesterolemia. However, the other chemical ingredients in OS leaves might participate in lipid-lowering and cardiac protective actions of OS leaves, and hence should not be excluded.
Among the chemical constituents contained in EO, eugenol, a phenolic compound, may be an active ingredient contributing for EO’s actions. This is supported by its action to lower high serum lipid profile in hyperlipidemic mice [22]. Moreover, eugenol has been shown to express antioxidant activity as detected by the DPPH assay [23]. Eugenol also attenuated isoproterenol-induced cardiac apoptosis [24]. It has been known that oxidation of low-density lipoprotein (LDL) is an important risk factor for atherosclerosis development [25]. LDL oxidation was increased in rabbit fed with HC diet for 8 weeks [26]. Eugenol has been found to act as an antioxidant to inhibit LDL oxidation and reduce lipid peroxidation [27, 28]. Its mechanism of action has been proposed by Ito et al. [28]. Eugenol inhibited iron-mediated lipid peroxidation and copper dependent LDL oxidation. Though EO contains a lot of methyl eugenol, there are no supporting data to show its anti-lipidemic and antioxidative effect.
Conclusions
In summary, EO was able to decrease the serum lipid profile and AI levels as well as cardiac lipid peroxidation and significantly enhanced the activity of the cardiac antioxidant enzymes. Our data indicate that EO treatment was very successful in preventing atherosclerosis and providing cardiac protection against hypercholesterolemia. The active ingredient eugenol contained in EO may play an important role for these therapeutic effects.
Acknowledgment
This project was supported by the grant from The Thailand Research Fund, Thailand (RMU 5080016).
References
- 1.Freedman J.E. High-fat diets and cardiovascular disease. J. Am. Col. Cardiol. 2003;41:1750–1752. doi: 10.1016/s0735-1097(03)00303-6. [DOI] [PubMed] [Google Scholar]
- 2.Khoo K.L., Tan I.T., Liew Y.M., Deslypere J.P., Janus E. Lipids and coronary heart disease in Asia. Atherosclosis. 2003;169:1–10. doi: 10.1016/s0021-9150(03)00009-1. [DOI] [PubMed] [Google Scholar]
- 3.Donahue R.P., Abbott R.D., Bloom E., Reed D.M. Central obesity and coronary heart disease in men. Lancet. 1987;11:821–824. doi: 10.1016/s0140-6736(87)91605-9. [DOI] [PubMed] [Google Scholar]
- 4.Kwiterovich P.O. The effect of dietary fat, antioxidants, and pro-oxidants on blood lipids, lipoproteins, and atherosclerosis. J. Am. Diet. Assoc. 1997;97:S31–S41. doi: 10.1016/s0002-8223(97)00727-x. [DOI] [PubMed] [Google Scholar]
- 5.Tribble D.L. Antioxidant consumption and risk of coronary heart disease: emphasis on vitamin C, vitamin E, and β-carotene: A statement for healthcare professionals from the American Heart Association. Circulation. 1999;99:591–595. doi: 10.1161/01.cir.99.4.591. [DOI] [PubMed] [Google Scholar]
- 6.Vijayakumar R.S., Surya D., Nalini N. Antioxidatant efficiency of black pepper (Piper nigrum L.) and piperine in rats with high fat diet induced oxidative stress. Redox Rep. 2004;9:105–110. doi: 10.1179/135100004225004742. [DOI] [PubMed] [Google Scholar]
- 7.Verlangieri A.J., Kapeghian J.C., El-Dean S., Bush M. Fruit and vegetable consumption and cardiovascular mortality. Med. Hypotheses. 1985;16:7–15. doi: 10.1016/0306-9877(85)90035-0. [DOI] [PubMed] [Google Scholar]
- 8.Suanarunsawat T., Songsak T. Anti-hyperglycemic and anti-hyperlipidemic effect of dietary supplement of white Ocimum sanctum Linn. before and after STZ-induced diabetes mellitus. Int. J. Diabetes & Metabolism. 2005;13:18–23. [Google Scholar]
- 9.Sharma M.K., Kumar M., Kumar A. Ocimum sanctum leaves extract provides protection against mercury induced toxicity in Swiss albino mice. Indian J. Exp. Biol. 2002;40:1072–1082. [PubMed] [Google Scholar]
- 10.Sood S., Narang D., Dinda A.K., Maulik S.K. Chronic oral administration of Ocimum sanctum Linn. augments cardiac endogenous antioxidants and prevents isoproterenal-induced myocardial necrosis in rats. J. Pharma. Pharmacol. 2005;57:127–133. doi: 10.1211/0022357055146. [DOI] [PubMed] [Google Scholar]
- 11.Ohgawa H., Ohishi N., Yaki K. Assay for lipid peroxide in animal tissues by Thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 12.Lowry O.H., Rosebrough N.J., Farr A.L., Randall J. Protein measurement with Folin phenol reagent. J. Biol. Chem. 1954;193:265–275. [PubMed] [Google Scholar]
- 13.Tapple A.L. In: Glutathione peroxidase and hydroperoxidase methods, in Methods in Enzymology, Vol II. Sidney F., Lester P., editors. Academic Press; New York: 1978. pp. 506–513. [Google Scholar]
- 14.Luck H. In: Catalase, in Method for Enzymatic Analysis, Vol. 3. H.-U. Bergmeyer., editor. Academic Press; New York and London: 1965. pp. 885–888. [Google Scholar]
- 15.Winterbourn C.C., Hawkings R.E., Brain M., Canell R.W. The estimation of red cell superoxide dismutase activity. J. Lab. Clin. Med. 1975;85:337–341. [PubMed] [Google Scholar]
- 16.Humanson G.L. In: Part I Basic procedure, in Animal tissue technique, 4th ed. W.H. Freeman, company , editor. San Francisco: 1979. pp. 1–129. [Google Scholar]
- 17.Braunwald E. Shattuck lecture-cardiovascular medicine at the turn of the millennium: triumps, concerns, and opporturnities. N. Eng. J. Med. 1997;337:1360–1369. doi: 10.1056/NEJM199711063371906. [DOI] [PubMed] [Google Scholar]
- 18.Anderson T.J., Meredith I.T., Yeung A.C., Frei B., Selwyn A.P., Ganz P. The effect of cholesterol-lowering and antioxidant therapy on endothelium-dependent coronary vasomotion. N. Eng. J. Med. 1995;332:488–493. doi: 10.1056/NEJM199502233320802. [DOI] [PubMed] [Google Scholar]
- 19.Bhatnagar D. Lipid-lowering drugs in management of hyperlipidemia. Pharmacol. Therapeut. 1998;79:205–230. doi: 10.1016/s0163-7258(98)00018-7. [DOI] [PubMed] [Google Scholar]
- 20.Manach C., Williamson G., Morandm C., Scalbert A., Remesy C. Bioaviailability and bioefficacy of polyphenols in human: Review of 97 bioavaibility studies. Am. J. Clin. Nutr. 2005;8:230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 21.Suanarunsawat T., Wacharaporn D.N.A., Songsak T., Rattanamahaphoom J. Anti-lipidemic actions of essential oil extracted from Ocimum sanctum L. leaves in rats fed with high cholesterol diet. J. Appl. Biomed. 2009;7:45–53. [Google Scholar]
- 22.Germán C., Leticia G., Adrián S., Fermando L., Maria S., Elizdath M., Francisco D., Joaquin T. Hypolipidemic activity of dimethoxy unconjugated propenyl side-chain analogs of α-asarone in mice. Drug Dev. Res. 1998;43:105–108. [Google Scholar]
- 23.Teresa M., Trevison S., Goretti M., Silva V., Pfundstein B., Spiegelhalder B., Owen R.W. Characterization of the volatile pattern and antioxidant capacity of essential oils from different species of the genus Ocimum. J. Agric. Food Chem. 2006;54:4378–4382. doi: 10.1021/jf060181+. [DOI] [PubMed] [Google Scholar]
- 24.Choudhary R., Mishra K.P., Subramanyam C. Interrelations between stress and calcineurin in the attenuation of cardiac apoptosis by eugenol. Mol. Cell Biochem. 2006;283:115–122. doi: 10.1007/s11010-006-2386-3. [DOI] [PubMed] [Google Scholar]
- 25.Steinberg D. Role of oxidized LDL and antioxidants in atherosclerosis. Adv. Exp. Med. Biol. 1995;369:39–48. doi: 10.1007/978-1-4615-1957-7_5. [DOI] [PubMed] [Google Scholar]
- 26.Özsoy M.B., Pabuçcuoğlu A. The effect of acetaminophen on oxidative modification of low-density lipoproteins in hypercholesterolemic rabbit. J. Clin. Biochem. Nutr. 2007;41:27–31. doi: 10.3164/jcbn.2007004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teissedre P.L., Waterhouse A.L. Inhibition of oxidation of human low density lipoproteins by phenolic substances in different essentials varieties. J. Agric. Food Chem. 2000;48:3801–3805. doi: 10.1021/jf990921x. [DOI] [PubMed] [Google Scholar]
- 28.Ito M., Murakami K., Yoshino M. Antioxidant action of eugenol compounds: role of metal ion in the inhibition of lipid peroxidation. Food Chem. Cell Toxicol. 2005;43:461–466. doi: 10.1016/j.fct.2004.11.019. [DOI] [PubMed] [Google Scholar]