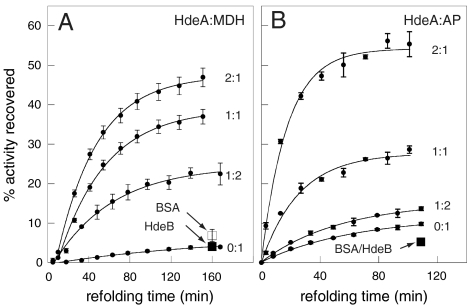
Fig. 2.
HdeA facilitates the refolding of acid-denatured substrates to an enzymatically active state. (A) 1 μM MDH or (B) 3 μM AP were incubated in the absence or in the presence of increasing concentrations of HdeA in buffer A (pH 2) for 1 h at 37 °C prior to equilibration at 20 °C and pH neutralization. Aliquots were taken at time points following pH neutralization and assayed in triplicate for MDH or AP activity. Error bars are ± 1 s.d. The effect of a twofold molar excess of BSA (□) and HdeB (▪) on MDH and AP refolding were also tested.