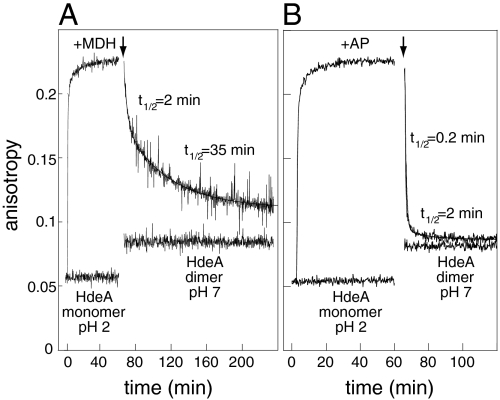
Fig. 3.
Substrate binding and release kinetics as monitored by fluorescence anisotropy. Anisotropy of 0.5 μM HdeA (S27C)-bimane at pH 2 in the absence or presence of 0.5 μM (A) MDH or (B) AP (added at t = 1 min) was monitored for 60 min at 37 °C. The temperature was then adjusted to 20 °C for 5 min, during which time data acquisition was paused. HdeA inactivation and substrate release were then triggered by pH neutralization (as indicated by the arrows). The traces corresponding to substrate release after neutralization were fit to double exponential functions; fit parameters: (A) A1 = 0.055, k1 = 0.30 min-1, A2 = 0.066, k2 = 0.020 min-1 and (B) A1 = 0.14, k1 = 4.3 min-1, A2 = 0.03, k2 = 0.29 min-1.