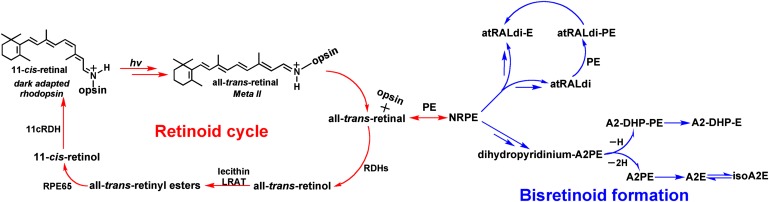
Fig. 1.
Retinoid cycling in the eye. The visual chromophore 11-cis-retinal forms a covalent Schiff base bond with lysine 296 (Lys 296) of opsin. Vision is initiated when a photon (hv) is captured by 11-cis-retinal; as a result, the chromophore is isomerized to all-trans-retinal. With all-trans-retinal still covalently bound to opsin, the activated pigment transitions to the metarhodopsin II conformation, the Schiff base is hydrolyzed, and all-trans-retinal is reduced to all-trans-retinol by retinol dehydrogenases (RDHs). Alternatively some all-trans-retinal reacts with phosphatidylethanolamine (PE) in the lipid bilayer to form N-retinylidene-PE, which is transported by ABCA4 and then hydrolyzes to release PE and all-trans-retinal. The latter is subsequently reduced to all-trans-retinol. Within the retinal pigment epithelium (RPE) cell, all-trans-retinol is esterified by the enzyme lecithin retinol acyl transferase (LRAT) and is isomerized from the all-trans configuration to the 11-cis-retinol by RPE65. The alcohol is then oxidized by 11-cis retinol dehydrogenase (11cRDH) to 11-cis-retinal. The bisretinoid pathway is initiated when N-retinylidene-PE, rather than hydrolyzing to all-trans-retinal and PE, reacts with a second molecule of all-trans-retinal. A multi-step pathway leads to formation of the intermediate dihydropyridinium-A2PE. Automatic oxidation of dihydropyridinium-A2PE with loss of two hydrogens (−2H) generates A2PE, the immediate precursor of A2E. Loss of one hydrogen (−H) generates A2-dihydropyridine-PE (A2-DHP-PE); phosphate hydrolysis of the latter produces A2-DHP-E. Via an alternative path, all-trans-retinal dimer forms from the condensation of two all-trans-retinal. Reaction all-trans-retinal dimer with PE with formation of a protonated Schiff base linkage generates all-trans-retinal dimer-PE (atRALdi-PE), and phosphate hydrolysis of the latter yields all-trans-retinal dimer-ethanolamine (atRALdi-E).