Abstract
Objective
Complex aneurysms such as fusiform and very small aneurysms (< 3 mm) are challenging in neurovascular and endovascular surgery. Author reports follow-up results of 9 cases treated by sole stent technique with pertinent literature review.
Methods
A retrospective study was made of 9 patients who were treated by sole stenting technique for cerebral aneurysm between January 2003 and January 2009. Two of them had fusiform aneurysm, 5 had very small aneurysm, and 2 had small saccular aneurysm. Five patients had ruptured aneurysms and four had unruptured aneurysms. Seven aneurysms were located in the internal carotid artery (ICA), 1 in the middle cerebral artery (MCA) and 1 in the basilar artery. Follow-up cerebral angiography was performed at post-procedure 3 months, 6 months, and 12 months. Mean follow-up period is 30 months (ranged from 3 days to 30 months).
Results
Aneurysm size was decreased in 6 of 9 cases on follow-up images and was not changed in 3 cases. Although total occlusion was not seen, patients had stable neurological condition and angiographic result. The procedural complication occurred in 2 cases. One was coil migration and the other was suboptimal deployment of stent, and both were asymptomatic. Re-bleeding and thromboembolic complication had not been occurred.
Conclusion
Sole stenting technique is relatively effective and safe as an alternative treatment for fusiform and very small aneurysms.
Keywords: Sole stenting technique, Fusiform aneurysm, Very small aneurysm
INTRODUCTION
Complex aneurysms such as very small aneurysms (< 3 mm) and fusiform aneurysm are challenging in neurovascular and endovascular surgery1,3-6,8,10,13,20,21,23,26,29,30,33). Although endovascular techniques and devices have developed remarkably, there are still limitations to treat complex aneurysm. Treatment for very small aneurysm is difficult because of tearing or narrowing the parent vessel during clipping and insufficient saccular space to deploy coil in endovascular treatment5,27,29). Fusiform aneurysm also is difficult and dangerous to be treated by surgical clipping or endovascular coiling because it has fragile wall and indistinct neck. In past series, the "deconstructive" methods, described as proximal occlusion or trapping, had been treatment of choice for fusiform aneurysm7,9,12). With the development of stent material and technique, the new concept has been evolved recently into "endovascular bypass" or "endovascular reconstruction" which is enabled to preserve affected parent artery and perforators, and occlude aneurysm safely. Compared with the deconstructive methods, the reconstructive ones may be more definitive and ideal treatment physiologically. Sole stenting technique is one of reconstructive methods and has been reported on alternative treatment of complex aneurysms, which were extremely difficult and dangerous to be deployed coils1,4,10,26). However, the effectiveness and safety of this technique had not been defined well. The complications such as thromboembolism associated with stenting, in-stent stenosis, and occlusion of perforating artery had been rarely reported3,10,11,19,22,30). We report follow-up results of 9 cases treated by sole stent technique, and review the results in other literatures.
MATERIALS AND METHODS
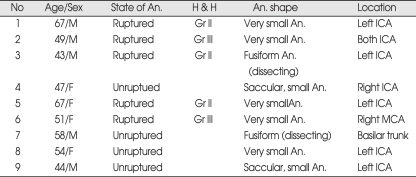
A retrospective study was made of 9 patients who treated by sole stenting technique for cerebral aneurysm between January 2003 and January 2009. After neurovascular and endovascular surgeons had discussed about methods of treatment for patients and concluded that the sole stent technique was best appropriate method, procedure was performed. Medical and radiological records were reviewed in all patients. Demographic factors of patients treated by sole stenting technique are shown in Table 1. There were 5 males and 4 female with mean age of 53.3 years (range from 43 to 67 years), and 5 cases were ruptured and 4 unruptured. Fusiform aneurysm was two in number, very small aneurysm (< 3 mm) in five, and small aneurysm (> 3 mm) in two. Seven aneurysms were located in the ICA, 1 in the middle cerebral artery (MCA) and 1 in the basilar artery.
Table 1.
Demographic factors of patients treated by sole stenting technique
An. : aneurysm, ICA : internal carotid artery, MCA : middle cerebral artery
In unruptured cases, dual antiplatelet therapy had been treated during 2-3 days before procedure. In ruptured cases, due to risk of re-bleeding, dual antiplatelet was not prescribed. At the beginning of the procedure, the patient was pretreated by heparin bolus (5,000 IU) injection, and followed by 1,000 IU/hour of heparin. Patient was heparinized to an activated clotting time (ACT) of 250 to 300 seconds during the procedure. After procedure, dual antiplatelet therapy (75 mg clopidogrel daily for 1month and 100 mg aspirin daily for 6 months) was prescribed in all cases.
Types of implanted stents were the Neuroform stent (Boston Scientific/Target Therapeutic, Fremont, CA, USA) in 7 cases, the Enterprise (Cordis Neurovascular, Miami, FL, USA) in 1 and both the Neuroform stent and the Enterprise in 1. Post-procedure cerebral angiography was obtained immediately. Follow-up cerebral angiography was performed at post-procedure 3 months, 6 months and 12 months. Mean follow-up period is 10 months (ranged from 3 days to 30 months).
RESULTS
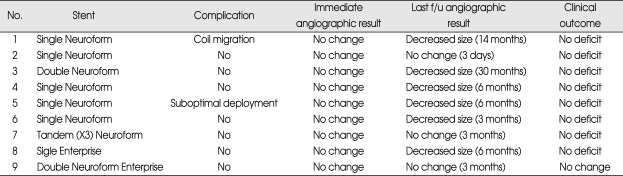
The results of patients are shown in Table 2. Eight patients were treated by sole stenting technique and 1 by stent assisted coiling. The latter patients (Case 1) had ruptured very small aneurysm of the left ICA. At 3 months after stent assisted coiling, the migration of coil mass into distal MCA was revealed in follow-up cerebral angiography. Fortunately, he had no clinical change and normal neurologic state. Due to very small size of aneurysm, further coil insertion could not be performed thus the patient was kept under careful observation with serial imaging follow-ups. Decreased size of aneurysm was shown in follow-up cerebral angiography at post-procedure 14 months and his neurologic state was normal (Fig. 1).
Table 2.
The outcome of patients treated by sole stenting technique
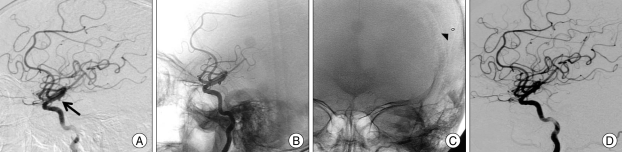
Fig. 1.
A 67-year-old male (Case 1) with a ruptured internal carotid artery (ICA) aneurysm. A : Preoperative left ICA angiogram in the lateral view shows a very small aneurysm of the ICA (Arrow indicates aneurysm). B : Left ICA angiogram in the same view immediately after stent assisted coil embolization. C : Follow-up image at postoperative 3 months demonstrates coil migration (arrow head). D : Follow-up ICA angiogram at postoperative 12 months shows decreased size of aneurysm.
Single stenting was performed in 6 cases, double stenting in 2, and tandem stenting was performed in 1, and a patient (Case 3) with fusiform dissecting aneurysm of the left ICA was treated by double stent insertion. He experienced subarachnoid hemorrhage and his neurologic state was Hunt and Hess grade 2 at admission. Double stenting technique with two Neuroform stent was performed and there were no procedural complications. Although there was no change of aneurysm size in immediate cerebral angiography, decreased aneurysm size and reconstruction of parent artery was revealed in follow-up angiography at post-procedure 6 months. In angiography at post-procedure 30 months, aneurysm size was not change and his neurologic state was normal (Fig. 2). Tandem stenting was performed in vertebrobasilar dissecting aneurysms (Case 7). Because the aneurysm affected in too long segment of basilar artery (50 mm), one or two stents were unable to cover the affected lesion. Therefore, patients underwent tandem stenting with three Neuroform stents. The procedural complications were not seen. Cerebral angiography at post-procedure 3 month showed no aneurysm growing and patient had stable neurological condition.
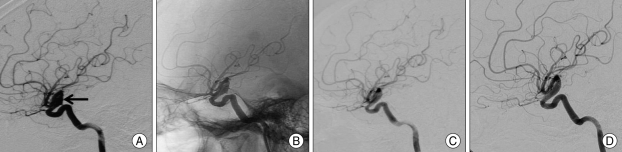
Fig. 2.
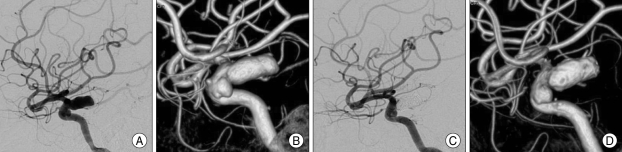
A 43-year-old male (Case 3) with dissecting aneurysm of the left internal carotid artery (ICA). A : Preoperative left ICA angiogram in the lateral view shows dissecting aneurysm (arrow) of the left ICA. B : Immediate postoperative angiogram in same view demonstrates inserted stents and no change of aneurysm. C : Follow-up angiogram at postoperative 6 months demonstrates decreased size of aneurysm and blunting of rupture site. D : At postoperative 30-month angiogram shows no change of aneurysm size.
One patient (Case 4) had two aneurysms which were small (3 × 5 mm) sized, saccular unruptured aneurysm in cavernous segment of the right ICA and a ruptured aneurysms in the communicating segment of same side ICA. The stent was deployed to cover the necks of ruptured and unruptured aneurysm, and coil embolization was only performed for the ruptured one. At post-operative 6 months, cerebral angiography showed that the size of unruptured aneurysm was decreased (Fig. 3).
Fig. 3.
A 47-year-old female (Case 4) with two aneurysms in the right internal carotid artery (ICA). A and B : Preoperative right ICA angiogram and 3 dimensional (3D) reconstruction view demonstrate two ICA aneurysms, one of them is the ruptured posterior communicating artery (PComA) aneurysm and the other is unruptured aneurysm in the cavernous segment of right ICA. C and D : Follow-up right ICA angiogram and 3D reconstruction view at postoperative 6 months show complete occlusion of PComA aneurysm and decreased size of the cavernous aneurysm.
In 6 of 9 cases, aneurysm size was decreased and in 3 cases, was not changed at follow-up images. Although total occlusion was not seen, all patients had stable neurological condition and angiographic result. The procedural complications occurred in 2 cases. One was coil migration into the distal artery and the other suboptimal deployment of stent. However, the complication was asymptomatic. Re-bleeding and thromboembolic complication had not been occurred.
DISCUSSION
The sole stenting technique in the treatment of cerebral aneurysm has developed as an alternative tool to stent assisted coil embolization and had occluded many complex aneurysms1,3-6,8,10,13,20,21,23,26,29,30,33). Their mechanism was explained as hemodynamic cause and thrombotic phenomenon. Implanted stent alters the flow pattern and reduces the flow into the aneurismal sac, and finally results in aneurismal thrombosis and occlusion. Additionally, the neointimal tissue covers throughout the luminal surface of the stent and the affected artery is reconstructed. Lopes and Sani18) reported that histological evaluation of the Neuroform stent 4 months after stenting showed complete endothelialization of the stent in autopsy, and de novo fibroblastic tissue formation along the neck of the aneurysm.
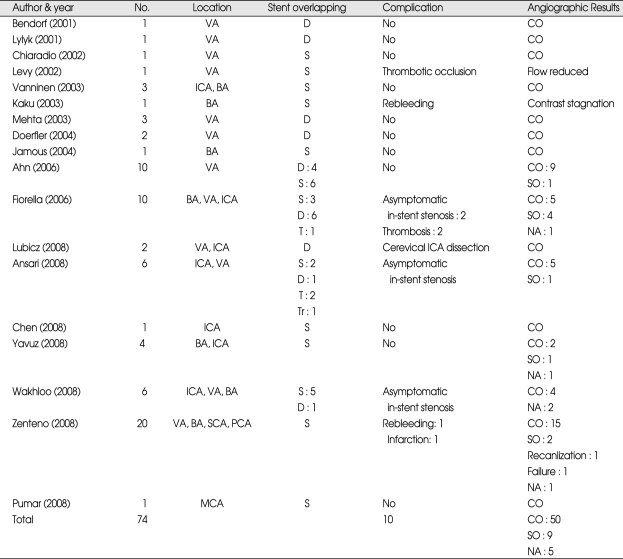
We reviewed the literatures on complex aneurysm treated by sole stenting technique and found 74 cases in 18 articles (Table 3). Fifty-nine of 74 cases (79.7%) resulted in total and stable subtotal occlusion at follow-up cerebral angiography. Five of 74 cases (6.8%) showed procedure related symptomatic complications. Four cases (5.4%) showed asymptomatic in-stent stenosis. In our series, 6 patients (66%) experienced subtotal occlusion and there are no symptomatic procedure related complications. Author considers that these results are favorable.
Table 3.
Summary of literature associated with sole stenting technique
BA : basilar artery, CO : Complete occlusion, D : double stent, ICA : internal carotid artery, NA: not available, PCA: posterior cerebellar artery, S : single stent, SO : subtotal occlusion, SCA : superior cerebellar artery, T : triple stent, Tr : Tetra stent, VA: vertebral artery
The effectiveness of stent overlapping has been controversy. Kim et al.16) reported hemodynamic influence according to number of stents, and that double stent model had more significant change. In contrast, Rhee et al.27) suggested no significant effect related on porosity. The number of overlapping stents was usually determined by surgeon's preference. Endovascular surgeons who prefer stent overlapping suggested that stent overlapping could decrease the porosity of stent construct and decrease the inflow to the aneurysm, and thus enhancing blood stasis and thrombosis4,8,20,21,23). However, some endovascular surgeons had experienced good results from single stenting1,5,6,13,26,29,33). To correlate between hemodynamic change and stent's porosity, further investigation should be necessary.
Fusiform dissecting aneurysm and sole stent
Incidence of intracranial fusiform aneurysms is rare (< 0.1%)25). Nevertheless, the fusiform aneurysm, especially dissecting aneurysm, is extremely fatal condition and mortality rate in 5 years is up to 80% in ruptured cases28). Intracranial arterial dissections can cause an ischemic stroke or devastating hemorrhage1,2,4,6,13,14,20,21,23,26,30). Presenting symptoms are various and are associated with pathologic process such as mass effect, ischemic event from thromboembolism or hemorrhage. In cases of vertebral artery dissection, the rebleeding rate is extremely high (> 30%)2,24). Due to fragile aneurysm wall, deep location, circumferential shape and incorporated perforators, fusiform aneurysms are too difficult and dangerous to be treated by clip or coil. In past years, proximal occlusion (or trapping) had been treatment of choice7,9,12). These deconstructive techniques are risky procedure itself and may need rigorous preoperative examination (etc balloon occlusion test), and if collateral circulation was insufficient, more complex procedure such as bypass surgery might be necessary. Also, retrograde flow may be a cause of aneurysm re-growth. At present, many endovascular surgeons has treated it by reconstructive method such as stent technique and experienced good result. Author believes that stent technique with or without coiling is more physiologic method for treatment of fusiform aneurysm.
Very small saccualr aneurysm and sole stent
In some cases, neurovascular surgeons meet very small aneurysm including blood blister like aneurysm, which is extremely dangerous to treat by using clip or coiling5,27,29). Chen et al.5) reported very small aneurysm of supraclinoid ICA treated by sole stenting technique. Aneurysm obliterated completely at 8 month after stenting. Vanninen et al.29) described 3 cases of saccular aneurysm which were successfully treated with coronary stent alone. They considered that small aneurysm may be obliterated easier than large ones. The sole stenting technique may bring on spontaneous aneurismal thrombosis gradually during the following weeks when aneurysm was extremely dangerous to treat by coiling27). Author had treated 5 case of very small aneurysm by sole stenting technique, 4 cases of them achieved decreased size reduction and all of them had stable clinical state. Size reduction of small aneurysm in the cavernous ICA after stenting in remaining one case. It is thus considered that sole stent technique may be an alternative treatment method for very small and small aneurysm, which is not amenable with conventional treatment.
The complications related to intracranial stenting are in-stent stenosis, thrombosis and thromboembolic event3,10,11,17,19,22,33).
In-stent stenosis
Although in-stent stenosis is major problem on stenting for artherosclerotic stenosis, parent artery of aneurysm is generally non-artheromatous and non-stenotic, and stent has low radial force, so in-stent stenosis on the treatment for aneurysm occurs rarely3,10,11,30). Some authors3,11) had reported asymptomatic in-stent stenosis, which resolved spontaneously on follow-up angiography. They suggested conservative treatment such as dual antiplatelet therapy, follow-up image and neurologic examination in patient with asymptomatic in-stent stenosis. Fiorella et al.11) reported 5.8% rate of moderate to severe (> 50%) in-stent stenosis and 1.3% rate of symptomatic stenosis. Prior to self-expandable, low radial forced intracranial stent was developed, intracranial stenting had been performed by coronary stent, which was balloon-mounted and has high radial force. High radial force was the cause of in-stent stenosis. Neuroform stent was one of intracranial stent, which characterized as self-expandable, open cell design, and ultra-thin strut. Some authors3,11,30) suggested that due to lower radial force of Neuroform stent, in-stent stenosis might be occurred rarer than coronary stent.
Perforator patency
Occlusion of perforator is one of major complications from intracranial stenting19,22). Three main mechanisms include snow plowing effect, stent jailing, and in-stent neointimal hyperplasia19). Snow plowing effect occurs from longitudinal redistribution of artherosclerotic plaque by stenting. Prior to stent insertion, meticulous evaluation about artherosclerotic parent vessels may be necessary. Stent jailing occurs when stent strut covered the ostium of perforators. Wakhloo et al.31) suggested that perforators might tend to remain patent below 50% of the ostial diameter covered by the struts. Masuo et al.22) investigated experimental in vivo model, and suggested that intracranial stenting may not occlude perforating arteries of the same diameter, even if stent struts cover the ostium. Author suggests that careful evaluation of artherosclerosis, use of low profile stent, and dual antiplatelet therapy are necessary for preventing occlusion of perforators. Also, because Neuroform stent has ultrathin strut and low radial force, perforator occlusion rate may be very low.
Thrombosis and dual antiplatelet therapy
Major disadvantage of intracranial stenting is thrombogenesity. Many clinicians10,11,17,30,33) have recommended pretreatment of dual antiplatelet therapy especially in cases of unruptured aneurysm. However, in ruptured cases, antiplatelet therapy may be the cause of premature rupture. Kim15) reported the low rate of thromboembolic event without premedication of antiplatelets regimen and suggested that premedication of dual antiplatelet therapy did not seem necessary. After treatment of aneurysm with stenting, dual antiplatelet therapy should be prescribed to prevent thromboembolic complications. Appropriate periods of times are necessary until aneurysm is occluded completely. During these periods, the risk of hemorrhage may increase because of dual antiplatelet therapy. Author's patients treated by stent alone had not experienced additional cerebral hemorrhage. To our experience, dual antiplatelet therapy may not increase hemorrhagic risk. As the regimen of dual antiplatelet therapy, combination clopidogrel (75 mg for 1 month) with aspirin (100 mg for 6 month) was prescribed to patients in author's hospital.
CONCLUSION
Although there were no cases of complete occlusion, 9 patients treated by sole stenting technique have stable clinical state and favorable angiographic result. Sole stenting technique can be a relatively effective and safe alternative in the treatment of fusiform and very small sized aneurysm, which are not amenable with conventional surgical or endovascular therapy. More long-term outcome and larger analysis should be necessary.
Acknowledgements
The present research was conducted under a research fund from Dankook University in 2008.
References
- 1.Ahn JY, Han IB, Kim TG, Yoon PH, Lee YJ, Lee BH, et al. Endovascular treatment of intracranial vertebral artery dissections with stent placement or stent-assisted coiling. Am J Neuroradiol. 2006;27:1514–1520. [PMC free article] [PubMed] [Google Scholar]
- 2.Albuquerque FC, Fiorella DJ, Han PP, Deshmukh VR, Kim LJ, McDougall CG. endovascular management of intracranial vertebral artery dissecting aneurysms. Neurosurg Focus. 2005;18:E3. [PubMed] [Google Scholar]
- 3.Ansari SA, Thompson BG, Gemmete JJ, Gandhi D. Endovacular treatment of distal cervical and intracranial dissection with the neuroform stent. Neurosurgery. 2008;62:636–646. doi: 10.1227/01.NEU.0000311350.25281.6B. [DOI] [PubMed] [Google Scholar]
- 4.Benndorf G, Herbon U, Sollmann WP, Campi A. Treatment of a ruptured dissecting vertebral artery aneurysm with double stent placement : case report. Am J Neuroradiol. 2001;22:1844–1848. [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Z, Feng H, Tang W, Liu Z, Miao H, Zhu G. Endovascular treatment of very small intracranial aneurysms. Surg Neurol. 2008;70:30–35. doi: 10.1016/j.surneu.2007.05.059. [DOI] [PubMed] [Google Scholar]
- 6.Chiaradio JC, Guzman L, Padilla L, Chiaradio MP. Intravascular graft stent treatment of a ruptured fusiform dissecting aneurysm of the intracranial vertebral artery : technical case report. Neurosurgery. 2002;50:213–216. doi: 10.1097/00006123-200201000-00034. [DOI] [PubMed] [Google Scholar]
- 7.Debrun G, Fox AJ, Drake CG, Peerless S, Girvin J, Ferguson G. Giant unclippable aneurysm : treatment with detachable balloons. Am J Neuroradiol. 1981;2:167–173. [PMC free article] [PubMed] [Google Scholar]
- 8.Doerfler A, Wanke I, Egelhof T, Stolke D, Forsting M. Double-stent method : therapeutic alternative for small wide-necked aneurysms. Technical note. J Neurosurg. 2004;100:150–154. doi: 10.3171/jns.2004.100.1.0150. [DOI] [PubMed] [Google Scholar]
- 9.Drake C, Peerless S. Giant fusiform intracranial aneurysm : review of 120 patients treated surgically from 1965-1992. J Neurosurg. 1997;87:141–162. doi: 10.3171/jns.1997.87.2.0141. [DOI] [PubMed] [Google Scholar]
- 10.Fiorella D, Albuquerque FC, Deshmukh VR, Woo HH, Rasmussen PA, Masaryk TJ, et al. Endovascular reconstruction with the Neuroform stent as monotherapy for the treatment of uncoilable intradural pseudoaneurysms. Neurosurgery. 2006;59:291–300. doi: 10.1227/01.NEU.0000223650.11954.6C. [DOI] [PubMed] [Google Scholar]
- 11.Fiorella D, Albuquerque FC, Woo H, Rasmussen PA, Masaryk TJ, McDougall CG. Neuroform in-stent stenosis : incidence, natural history, and treatment strategies. Neurosurgery. 2006;59:34–42. doi: 10.1227/01.NEU.0000219853.56553.71. [DOI] [PubMed] [Google Scholar]
- 12.Gobin YP, Viñuela F, Gurian JH, Guglielmi G, Duckwiler GR, Massoud TF, et al. Treatment of large and giant fusiform intracranial aneurysms with Guglielmi detachable coils. J Neurosurg. 1996;84:55–62. doi: 10.3171/jns.1996.84.1.0055. [DOI] [PubMed] [Google Scholar]
- 13.Jamous MA, Satoh K, Matsubara S, Satomi J, Nakajima N, Uno M, et al. Ischemic basilar artery dissecting aneurysm treated by stenting only. Case report. Neurol Med Chir (Tokyo) 2004;44:77–81. doi: 10.2176/nmc.44.77. [DOI] [PubMed] [Google Scholar]
- 14.Kaku Y, Yoshimura S, Yamakawa H, Sakai N. Failure of stent-assisted endovascular treatment for ruptured dissecting aneurysms of the basilar artery. Neuroradiology. 2003;45:22–26. doi: 10.1007/s00234-002-0903-y. [DOI] [PubMed] [Google Scholar]
- 15.Kim YJ. Early experiences of neruoform stent-assisted coiling in ruptured intracranial aneurysms. Interv Neuroradiol. 2007;13:31–44. doi: 10.1177/159101990701300104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim M, Levy EI, Meng H, Hopkins LN. Quantification of hemodynamic changes induced by virtual placement of multiple stents across a wide-necked basilar trunk aneurysm. Neurosurgery. 2007;61:1305–1312. doi: 10.1227/01.NEU.0000280168.25968.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levy EI, Boulos AS, Bendok BR, Kim SH, Qureshi AI, Guterman LR, et al. Brainstem infarction after delayed thrombosis of a stented vertebral artery fusiform aneurysm : case report. Neurosurgery. 2002;51:1280–1284. doi: 10.1097/00006123-200211000-00026. [DOI] [PubMed] [Google Scholar]
- 18.Lopes D, Sani S. Histological postmortem study of an internal carotid artery aneurysm treated with the Neuroform stent. Neurosurgery. 2005;56:E416. doi: 10.1227/01.neu.0000147977.07736.66. [DOI] [PubMed] [Google Scholar]
- 19.Lopes DK, Ringer AJ, Boulos AS, Qureshi AI, Lieber BB, Guterman LR, et al. Fate of branch arteries after intracranial stenting. Neurosurgery. 2003;52:1275–1278. doi: 10.1227/01.neu.0000064567.15270.27. [DOI] [PubMed] [Google Scholar]
- 20.Lubicz B, Collignon L, Lefranc F, Bruneau M, Brotchi J, Balériaux D, et al. Circumferential and fusiform intracranial aneurysms : reconstructive endovascular treatment with self-expandable stents. Neuroradiology. 2008;50:499–507. doi: 10.1007/s00234-008-0366-x. [DOI] [PubMed] [Google Scholar]
- 21.Lylyk P, Cohen JE, Ceratto R, Ferrario A, Miranda C. Combined endovascular treatment of dissecting vertebral artery aneurysms by using stents and coils. J Neurosurg. 2001;94:427–432. doi: 10.3171/jns.2001.94.3.0427. [DOI] [PubMed] [Google Scholar]
- 22.Masuo O, Terada T, Walker G, Tsuura M, Matsumoto H, Tohya K, et al. Study of the patency of small arterial branches after stent placement with an experimental in vivo model. Am J Neuroradiol. 2002;23:706–710. [PMC free article] [PubMed] [Google Scholar]
- 23.Mehta B, Burke T, Kole M, Bydon A, Seyfried D, Malik G. Stent-within-a-stent technique for the treatment of dissecting vertebral artery aneurysms. Am J Neuroradiol. 2003;24:1814–1818. [PMC free article] [PubMed] [Google Scholar]
- 24.Mizutani T, Kojima H, Asamoto S, Miki Y. Recurrent subarachnoid hemorrhage from untreated ruptured vertebrobasilar dissecting aneurysms. Neurosurgery. 1995;36:905–913. doi: 10.1227/00006123-199505000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Mohr JP, Choi D, Grotta J, Wolf P. Stroke : Pathophysiology, Diagnosis, and Management. 4th ed. Philadelphia: Churchill Livingson; 2004. p. 1318. [Google Scholar]
- 26.Pumar JM, Lete I, Pardo MI, Vázquez-Herrero F, Blanco M. LEO stent monotherapy for the endovascular reconstruction of fusiform aneurysms of the middle cerebral artery. Am J Neuroradiol. 2008;29:1775–1776. doi: 10.3174/ajnr.A1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rhee K, Han MH, Cha SH. Changes of flow characteristics by stenting in aneurysm models : influence of aneurysm geometry and stent porosity. Ann Biomed Eng. 2002;30:894–904. doi: 10.1114/1.1500406. [DOI] [PubMed] [Google Scholar]
- 28.Steinberg GK, Drake CG, Peerless SJ. Deliberate basilar or vertebral artery occlusion in the treatment of intracranial aneurysms. Immediate results and long-term outcome in 201 patients. J Neurosurg. 1993;79:161–173. doi: 10.3171/jns.1993.79.2.0161. [DOI] [PubMed] [Google Scholar]
- 29.Vanninen R, Manninen H, Ronkainen A. Broad-based intracranial aneurysms : thrombosis induced by stent placement. Am J Neuroradiol. 2003;24:263–266. [PMC free article] [PubMed] [Google Scholar]
- 30.Wakhloo AK, Mandell J, Gounis MJ, Brooks C, Linfante I, Winer J, et al. Stent-assisted reconstructive endovascular repair of cranial fusiform atherosclerotic and dissecting aneurysms : long-term clinical and angiographic follow-up. Stroke. 2008;39:3288–3296. doi: 10.1161/STROKEAHA.107.512996. [DOI] [PubMed] [Google Scholar]
- 31.Wakhloo AK, Tio FO, Lieber BB, Schellhammer F, Graf M, Hopkins LN. Self-expanding nitinol stents in canine vertebral arteries : hemodynamics and tissue response. Am J Neuroradiol. 1995;16:1043–1051. [PMC free article] [PubMed] [Google Scholar]
- 32.Yavuz K, Geyik S, Saatci I, Cekirge HS. WingSpan Stent System in the endovascular treatment of intracranial aneurysms : clinical experience with midterm follow-up results. J Neurosurg. 2008;109:445–453. doi: 10.3171/JNS/2008/109/9/0445. [DOI] [PubMed] [Google Scholar]
- 33.Zenteno MA, Santos-Franco JA, Freitas-Modenesi JM, Gómez C, Murillo-Bonilla L, Aburto-Murrieta Y, et al. Use of the sole stenting technique for the management of aneurysms in the posterior circulation in a prospective series of 20 patients. J Neurosurg. 2008;108:1104–1118. doi: 10.3171/JNS/2008/108/6/1104. [DOI] [PubMed] [Google Scholar]