Table 2.
Alkylation of Electron-Rich Indole Substrates[a]
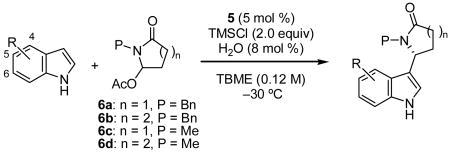 | ||||||
|---|---|---|---|---|---|---|
| Entry | R (indole) | lactam | Time (h) | Product | Yield [%][b] | ee [%] |
| 1 | H | 6a | 24 | 2a | 90 | 93 (99)[c] |
| 2 | H | 6c | 24 | 2b | 93 | 86 |
| 3 | 4-Me | 6a | 24 | 2c | 86 | 95 |
| 4 | 5-Me | 6a | 24 | 2d | 79 | 90 (91)[c] |
| 5 | 5-CH2CH2 | 6a | 24 | 2e | 82 | 90 |
| 6 | 6-OMe | 6a | 24 | 2f | 80 | 80 (98)[b] |
| 7 | H | 6d | 48 | 2g | 60 | 92 |
| 8 | H | 6b | 48 | 2h | 70 | 93 |
| 9 | 5-OMe | 6b | 48 | 2i | 86 | 90 |
| 10 | 5-Me | 6b | 48 | 2j | 93 | 94 |
| 11 | 5-CH2CH2 | 6b | 48 | 2k | 92 | 91 |
| 12 | 6-OMe | 6b | 48 | 2l | 76 | 88 |
Unless noted otherwise, reactions were carried out on 0.3 mmol scale.
Isolated yield after chromatographic purification.
Reaction carried out on 3 mmol scale.
Ee of product purified by trituration with Et2O in parentheses.
