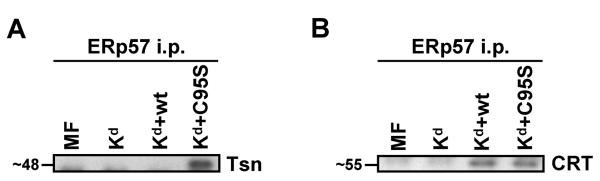
Figure 2.
Mouse tapasin C95S was discovered to bind non-covalently with ERp57, and both wild type and C95S tapasin were found to bind only non-covalently with calreticulin. (A) Immunoprecipitations with an antiserum against mouse ERp57 were performed on lysates of MMTS-treated MF cells transfected with Kd alone or Kd plus wild type tapasin or tapasin C95S. The immunoprecipitates were electrophoresed on a 10% acrylamide Tris-glycine gel and probed on a Western blot with a monoclonal antibody against mouse tapasin. (B) In MF cells, calreticulin was discovered to interact with ERp57 in the presence of wild type or C95S tapasin, but not in the absence of tapasin. ERp57 was immunoprecipitated from the indicated MMTS-treated cell lysates with anti-ERp57 antiserum, electrophoresed on a 10% acrylamide Tris-glycine gel, and associated calreticulin was detected by Western blotting with an anti-calreticulin antibody.