Abstract
• Background and Aims Fertilization is essential in almond production, and pollination can be limiting in production areas. This study investigated stigma receptivity under defined developmental stages to clarify the relationship between stigma morphology, pollen germination, tube growth and fruit set.
• Methods Light and scanning electron microscopy were employed to examine stigma development at seven stages of flower development ranging from buds that were swollen to flowers in which petals were abscising. Flowers at different stages were hand pollinated and pollen germination and tube growth assessed. Artificial pollinations in the field were conducted to determine the effect of flower age on fruit set.
• Key Results Later stages of flower development exhibited greater stigma receptivity, i.e. higher percentages of pollen germination and more extensive tube growth occurred in older (those opened to the flat petal stage or exhibiting petal fall) than younger flowers. Enhanced stigma receptivity was associated with elongation of stigmatic papillae and increased amounts of stigmatic exudate that inundated papillae at later developmental stages. Field pollinations indicated that the stigma was still receptive and nut set was maintained in older flowers.
• Conclusions Stigma receptivity in almond does not become optimal until flowers are past the fully open stage. The stigma is still receptive and fruit set is maintained in flowers even at the stage when petals are abscising. Strategies to enhance pollination and crop yield, including the timing and placement of honey bees, should consider the effectiveness of developmentally advanced flowers.
Keywords: Almond, effective pollination period, Prunus dulcis, stigma receptivity, stigmatic exudate
INTRODUCTION
Fertilization is essential for almond (Prunus dulcis) production (Cousin and El Maataoui, 1998). The crop is mainly self-incompatible and requires cross-pollination (Pimienta et al., 1983; Socias i Company et al., 2002). Pollination can be limiting in certain production areas, and it has been reported that the percentage of fruit set in commercial orchards is commonly only 30 % (Gary et al., 1976). Optimum pollination is critical for maximum crop production, and crop thinning is never practised (Connell, 2000).
The effective pollination period (EPP), first introduced by Williams in 1966, is one of the most important factors determining successful fertilization. EPP is determined by the longevity of the ovule minus the time lag between pollination and fertilization, providing that this value does not exceed the length of stigmatic receptivity. As reviewed by Sanzol and Herrero (2001), the EPP is limited by three main events during the reproductive process: stigma receptivity, pollen tube kinetics and ovule longevity.
Stigma receptivity refers to the ability of the stigma to support germination of viable, compatible pollen. It has been implicated as a factor limiting the EPP and fruit set in kiwifruit (Gonzalez et al., 1995b), apricot (Egea and Burgos, 1992), pear (Sanzol et al., 2003b) and cherry (Guerrero-Prieto et al., 1985; Furukawa and Bukovac, 1989). A short life span of ovules is limiting to EPP in sweet and sour cherries (Postweiler et al., 1985; Cerovic and Ruzic, 1992) and apricot (Burgos and Egea, 1993). Abnormalities of the ovule or embryo sac development limit EPP in olive (Rallo et al., 1981), avocado (Tomer et al., 1976) and almond (Pimienta and Polito, 1982). Unlike other Prunus species where well-developed embryo sacs are present at anthesis, almond ovules are in the megaspore-mother-cell stage at flower opening, and complete embryo sac maturation 7–8 d after anthesis (Pimienta and Polito, 1983). Since embryo sac development is stimulated by the presence of compatible pollen tubes in the style and final elongation growth of the embryo sac is promoted by cross-pollination (Pimienta and Polito, 1983), ovule longevity in almond may be less limiting to EPP than in species attaining a mature embryo sac at anthesis.
Details of stigma receptivity have been studied in only a limited number of species (Shivanna, 2003). Optimal receptivity is variable and can be from a few hours after flower opening as in teak (Tangmitcharoen and Owens, 1997), to a few days after anthesis as in oak (Kalinganire et al., 2000) and Silene alba (Young and Gravitz, 2002). Although there are a few histological studies of ovule development in almond (Sterling, 1964; Pimienta and Polito, 1983), evaluations of stigma development and its relationship to stigma receptivity are lacking. The timing of pollination for adequate fruit set has been evaluated in almond to some extent, but solely on a time/day basis which precludes direct comparisons due to differences in flower maturation that occur with environment and location. Furthermore, the contribution of stigma receptivity to support pollen germination and tube growth is unknown. The objectives of this study were to investigate stigma receptivity under defined developmental stages and to clarify the relationship between stigma morphology, pollen germination and tube growth. Such information would allow a greater understanding of how factors affect the EPP in almond, and thereby provide information providing strategies to optimize pollination and increase fruit set.
MATERIALS AND METHODS
Plant material
Almond [P. dulcis (Mill.) D.A. Webb] budwood from ‘Nonpareil’ and ‘Padre’ cultivars was collected from a commercial orchard (Paramount Farming Co., Bakersfield, CA, USA), packed in coolers, and shipped by next-day mail to the laboratory in Georgia. Upon delivery, shoots were recut under water, placed with their bases in water, and held in a dark cold room at 7 °C for up to 7 d. Buds were forced as needed under light conditions at room temperature, and collected for histological evaluations. Flowers were also used in pollination studies to assess stigma receptivity, in which case flowers were emasculated before anther dehiscence. Pollen collected from ‘Sonora’ and ‘Fritz’ trees was used to pollinate ‘Nonpareil’ and ‘Padre’ flowers, respectively. In vitro germination tests (Yi et al., 2003b) were conducted to verify pollen viability. Pollen was inoculated into germination wells containing 12 % sucrose (w/v), 0·062 % CaNO3 (w/v) and 0·024 % boric acid (w/v). Germination percentages were 51 and 41 % for these two cultivars, respectively.
Flower stage descriptions
Buds and flowers were classified into seven developmental stages (Fig. 1): stage 1, bud swollen, no pink visible; stage 2, pink visible, petal tightly closed; stage 3, petals extended, but corolla still tubular with opening at tip of petals; stage 4, petals unfurling, individual petals cup-shaped and curved; stage 5, fully open stage, little or no curvature in petals, individual petals are predominantly planar; stage 6, flattened stage, petals are attached to the floral axis at 0 ° angle or reflexed; stage 7, petal fall stage, all or most petals abscised, stigma has not darkened in colour.
Fig. 1.
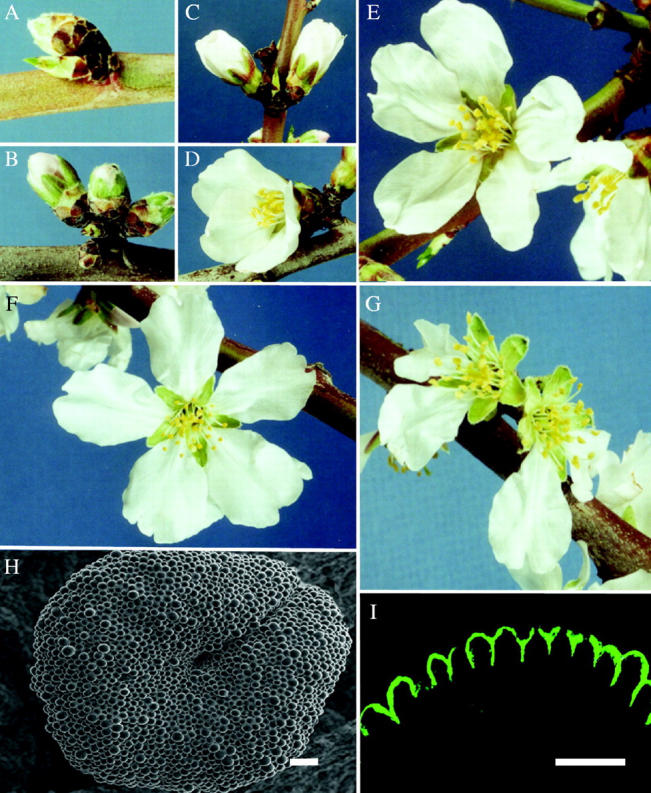
(A) Stage 1, swollen bud. (B) Stage 2, pink bud. (C) Stage 3, extended petals. (D) Stage 4, unfurling petals. (E) Stage 5, fully open. (F) Stage 6, flattened petals. (G) Stage 7, abscised petals. (H) SEM view of a stigmatic surface at stage 1. Scale bar = 80 µm. (I) Section through the surface of a stage 1 stigma stained with auramine O under fluorescence microscopy showing a cuticle layer on the surface. Scale bar = 40 µm.
Light microscopy
‘Nonpareil’ flowers from stages 1–7 were prepared for microscopic evaluations. Pistils were dissected and fixed in 2 % glutaraldehyde in cacodylate buffer pH 7·2, dehydrated in a series of methyl cellosolve (ethylene glycol monomethyl ether), ethanol, propanol and n-butanol, then infiltrated and embedded in Historesin (Leica Instruments, Heidelberg, Germany). Sections were cut using a HM350 rotary microtome (Microm, Heidelberg, Germany). For general histological observations, sections were stained with 0·05 % toluidine blue O, and examined using a Zeiss Standard microscope (Carl Zeiss, Oberkochen, Germany). Cuticles were localized using 0·01 % auramine O in Tris–HCl buffer (pH 7·2) under fluorescence illumination (Heslop-Harrison, 1977).
Scanning electron microscopy (SEM)
‘Nonpareil’ flowers at each stage were selected to observe as fresh, unfixed samples. Pistils were dissected from flowers/buds, and stigmas with attached styles were mounted on aluminium stubs using carbon paste. Each sample was observed immediately using a JSM-5800 scanning electron microscope (JEOL, Tokyo, Japan) at 5 kV. Images were captured digitally.
Pollination and tube growth assessment
Accurately counted numbers of pollen grains were applied onto the stigmas of flowers at selected developmental stages to assess pollen germination and tube growth. Direct counting of pollen numbers on the stigma was not possible because pollen is obscured by stigmatic exudate, thus pollen numbers were determined indirectly. Pollen grains were applied to the transparent covers of 12-well tissue culture plates (Costar Tissue Culture Clusters 12; Costar, Cambridge, MA, USA), and grain numbers were counted using an inverted microscope (Nikon, Garden City, NY, USA). Detached flowers were pollinated by gently touching the stigma to the pre-counted pollen grains. The number of pollen grains adhering to the stigma was determined by subtracting the number of remaining grains on the plate from the number of originally counted grains. In general, from 100 to 200 pollen grains were applied to each stigma. Flowers were transferred into the wells containing tap water and kept under light conditions at 24 °C.
At 24 h after pollination, flowers were dissected to remove pistils, then stigmas and styles were fixed in ethanol : acetic acid 3 : 1 (v/v). Tissues were softened and cleared by autoclaving at 120 °C for 20 min in 1 % sodium sulfite solution (w/v), stained using aniline blue (0·01 % aniline blue in 0·1 M K3PO4) for at least 4 h, then examined using a Zeiss Standard microscope (Carl Zeiss, Oberkochen, Germany) under fluorescent light (G365, LP420). The numbers of pollen tubes and the extent of their growth through the length of the style were assessed.
Four developmental stages (4, 5, 6 and 7) for each cultivar were assessed in the pollination and tube growth studies. The entire experiment was repeated three times at 2 d intervals with five flower replicates each time, using a completely randomized block design: four developmental stages × three blocks (days when the experiment was conducted) × five flower replications. Statistical analysis was conducted by GLM followed by Duncan's multiple range test at P=0·05 (SAS Institute, 1989).
Fruit set field studies
Fruit set of flowers hand pollinated at different developmental stages was assessed under commercial orchard conditions in Bakersfield, CA, USA in 2003. Investigations were carried out on 7-year-old ‘Butte’ and ‘Padre’ trees. To prevent insect pollination, individual branches within the middle third of the canopy were randomly selected and enclosed within fibreglass screen (1·6 mm opening) bags prior to flower opening. As branches came into bloom, they were assessed for uniformity and assigned a flower stage (stage 4, 5, 6 or 7) closest to the majority of their blooms. Flowers that were younger and older than the assigned stage were manually removed from branches at the time pollinations were made. Branches were successively pollinated throughout the bloom period of February 19 to March 7. Data from March 7 were excluded from the analysis, because all flowers pollinated on that date were infertile and did not set fruit.
The pollen used for hand pollinations was obtained from ‘Neplus’ trees and applied using a small paintbrush. The numbers of flowers on each branch were counted and bags were replaced until stigmas were desiccated shortly after petal fall. Young fruits were counted on May 5, and the percentage fruit set was calculated as the (number of fruit set)/(number of flowers pollinated) × 100 %. On August 7, nuts were harvested and dissected to assess the percentage of full nuts that were set as calculated by the (number of full nuts)/(number of flowers pollinated) × 100 %.
Thus, the experiment was blocked by pollination date with almond variety and flowering stage nested in a split plot design. A type 4 analysis of variance (ANOVA; SPSS, Inc.) was conducted since not all flowering stages were represented in each pollination date. Sample size was 40 after excluding one outlier (2·4 % of the data) as determined by Cooks Distance criterion.
RESULTS
Stigma development
Pistil morphology in almond is characterized by a single circular bilobed stigmatic surface (Fig. 1H) that expands slightly in a fan-like manner beyond an elongated and cylindrical style (Yi et al., 2003a). Stigmatic surface cells at stage 1 were composed of raised unicellular papillae (Fig. 2A and B). Sub-papillar regions were composed of small, densely staining cells (Fig. 2B). The short papillae were closely packed and had little visible exudate accumulation (Fig. 2A). A cuticle–pellicle layer on external surfaces of papillae was visualized with auramine O staining under fluorescence microscopy (Fig. 1I). At stages 2, 3 and 4, papillae were more elongated, and accumulated stigmatic exudate appeared at some interstitial regions at the base of adjacent papillae (Fig. 2C). In stages 2 and 3, stigmatic exudate accumulations were limited to the central region of stigmas, while in stage 4, exudate was observed in both central and peripheral areas. An intact cuticle was absent at these and subsequent stages. Elongated papillae were tightly packed, of uniform height and appeared turgid (Fig. 2D). With further elongation in stage 4, papillae had prominent vacuolar spaces.
Fig. 2.
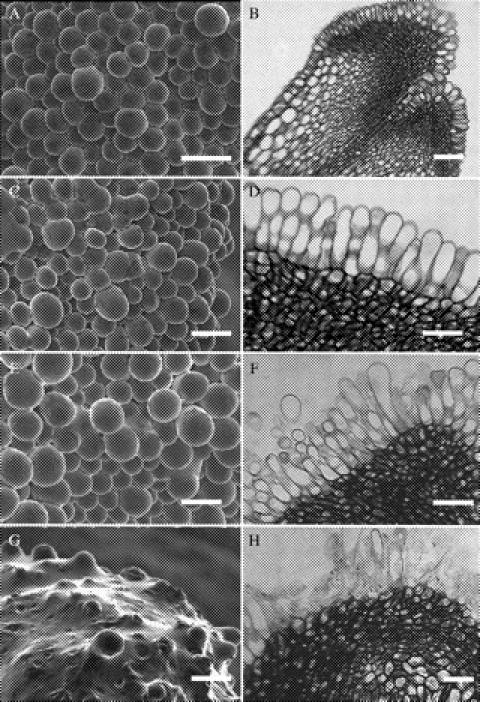
(A) Portion of a stage 1 stigmatic surface showing short papillae. Scale bar = 50 µm. (B) Longitudinal section through the stigma of a stage 1 flower. Scale bar = 75 µm. (C) Surface papillae from a stage 4 flower showing localized areas with exudate. Scale bar = 45 µm. (D) Light micrograph of a longitudinal section through a stage 4 stigma. Papillae are elongate with large vacuolar content. Scale bar = 50 µm. (E) Stage 6 stigma with some shorter papillae immersed in exudate. Scale bar = 40 µm. (F) Section through stage 6 stigma. Papillae have elongated differentially so that stigmatic cells are of different heights. Scale bar = 45 µm. (G) Surface view of a stage 7 stigma. Only a few elongated papillae are visible over the exudate. Scale bar = 40 µm. (H) Cross-section of a stage 7 stigma. Collapse of some papillae is evident. Scale bar = 41 µm.
Fully open flowers (stage 5) exhibited localized regions where exudate accumulated and could reach the tips of some papillae; minor collapse of some stigmatic cells occurred. Increasing amounts of exudate were observed as flowers developed further, as shown in stage 6 flowers where shorter papillae were partially inundated (Fig. 2E). Papillae elongated differentially so that stigmatic cells of variable heights were observed, with taller cells exhibiting enhanced protruding apical regions (Fig. 2F). Cells had more extensive vacuolar spaces which occupied most of the cell volume. In stage 7 flowers, which were initiated at the onset of petal fall, substantial and copious exudate submerged the entire stigmatic surface so that only a few elongated papillae were visible (Fig. 2G). Further collapse of papillae was observed (Fig. 2H).
Pollination studies
Flower stage had a significant and similar effect on pollen germination in both ‘Nonpareil’ and ‘Padre’ (Table 1). The percentage pollen germination was significantly higher in more developed flowers at stages 6 (flat petal) and 7 (petal fall) compared with younger flowers at stages 4 (petals unfurling) and 5 (fully open). The two cultivars differed in germination percentage, with ‘Padre’ flowers exhibiting up to approx. 15–20 % germination compared with ‘Nonpareil’ which had approx. 3–5 % germination. Younger staged ‘Nonpareil’ flowers failed to support pollen germination, i.e. germination was <0·4 %.
Table 1.
Pollen germination and tube growth in ‘Nonpareil’ and ‘Padre’ almond flowers, 24 h after pollination of flowers at different developmental stages with ‘Sonora’ and ‘Fritz’ pollen, respectively
| Germination percentage* |
Tube length (%)† |
|||
|---|---|---|---|---|
| Flower stage | ‘Nonpareil’ | ‘Padre’ | ‘Nonpareil’ | ‘Padre’ |
| Stage 4 | 0·1a‡ | 3·9a | 3a | 31ab |
| Stage 5 | 0·4a | 2·4a | 9ab | 22a |
| Stage 6 | 3·0b | 14·5b | 19b | 42bc |
| Stage 7 | 4·5b | 20·1b | 50c | 60c |
Germination percentage was calculated as (germinated pollen)/(viable pollen) × 100 %.
Length of longest pollen tubes as a percentage of the total length of the style.
Means within the same column followed by the same letters are not significantly different at P= 0·05 using Duncan's multiple range test.
Pollen tube growth was also impacted by flower developmental stage when the lengths of the longest pollen tubes were expressed as the percentage of the total style length that tubes penetrated (Table 1). In ‘Nonpareil’, the longest tube lengths were observed in flowers at stage 7, where at 24 h after pollination, tubes extended to 50 % of the style length. Maximum pollen tube length was significantly shorter in younger flowers after the same duration, where pollen extension at the two earliest stages was only 6–18 % of that obtained in the oldest stage flowers. In ‘Padre’, flower development had a less marked effect on tube extension. Although stage 7 flowers exhibited more extension tube growth than flowers at stages 4 or 5, maximum tube extension was over 30 % of the style length at even the youngest stage pollinated.
Fruit and nut set studies
Flowers hand pollinated at different developmental stages exhibited no significant differences in percentage fruit set among the four developmental stages (Table 2). Appreciable levels of fruit set were observed in flowers of all developmental stages for both ‘Butte’ (from 27 to 45 %) and ‘Padre’ (from 49 to 79 %). Similar results were observed in the percentage of full nuts (Table 2), with similar nut set observed in all stages. ‘Butte’ exhibited lower fruitfulness than ‘Padre’, obtaining levels of fruit and full nut set of only 35–70 % of that observed in ‘Padre’.
Table 2.
Fruit and nut set of ‘Butte’ and ‘Padre’ almond flowers after hand pollination at different developmental stages
| Fruit set (%)* |
Full nut set (%)† |
|||
|---|---|---|---|---|
| Flower stage | ‘Butte’ | ‘Padre’ | ‘Butte’ | ‘Padre’ |
| Stage 4 | 27·3a‡ | 78·9a | 27·3a | 74·7a |
| Stage 5 | 30·5a | 74·7a | 30·5a | 74·7a |
| Stage 6 | 45·1a | 70·9a | 44·2a | 67·8a |
| Stage 7 | 32·9a | 49·0a | 29·6a | 47·5a |
Fruit set percentage was calculated as: (number of fruit set)/(number of flowers pollinated) × 100 %.
Full nut percentage was calculated as: (number of full nut set)/(number of flowers pollinated) × 100 %.
Means within the same column followed by the same letters are not significantly different at P = 0·05 using Duncan's multiple range test.
DISCUSSION
The stigma is reported to be receptive at the time of anthesis in many tree crops such as peach, apricot, sweet cherry, apple and kiwi (Sanzol and Herrero, 2001). However, the receptive period can vary with species or cultivar, and requires delayed maturation of the stigma post-anthesis. Herrero (1983) reported that pear stigmas were not receptive at anthesis and pollen germination increased with time for 4 d. Likewise, stigmas in apricot were not mature at the balloon stage, but attained higher receptivity 2 and 4 d after flower opening (Egea et al., 1991). Williams et al. (1984) concluded that in apple, recently opened flowers were not yet fully receptive to pollination.
In the current study, stigma receptivity in ‘Nonpareil’ and ‘Padre’ almond flowers was delayed, with higher percentages of pollen germination and more extensive tube extension observed in older (flat petal or petal fall) than younger flowers (petals unfurling or fully open flowers). Previous studies to determine the optimal timing of stigma maturation in almond have resulted in variable responses depending on cultivar and environment. Ortega et al. (2004) pollinated almond flowers at 0, 2, 4 and 6 d after emasculation, and evaluated the number of pollen tubes in the style. Stigma receptivity varied with cultivar, and in some cases was optimal in youngest flowers and declined after 2 d; some cultivars were not receptive until 6 d after emasculation. Since flower stages at pollination were not defined, it is unclear if cultivars varied in flower development on their respective days after emasculation. Environmental conditions such as temperature at time of blooming may have impacted flower development and stigma receptivity.
Appreciable levels of fruit and nut set were observed in flowers pollinated at both younger (stage 4 and 5) and older (stage 6 and 7) stages, with no statistical differences observed. This is in contrast to our data on pollen tube numbers in the style, which indicate that younger flowers do not support pollen tube growth as well as older flowers. Almond flowering can progress rapidly under field conditions. We have observed flowers maturing from our stage 3 to stage 5 within a single day in California when temperatures are warm. Pollen grains can maintain their viability under room temperature conditions for a few days (data not shown). Therefore, pollen grains attached to young stigmas could germinate and fertilize flowers after undeveloped stigmas reach a sufficient maturity for pollen hydration, germination and tube growth to occur. This could explain the fruit set we obtained with young staged flowers that had low stigma receptivity. Although loss of ovule viability can impair fruit set if pollination occurs when flowers are too old, our data show that even flowers that exhibit petal abscission (stage 7) are capable of setting fruit at levels comparable with earlier staged flowers. At stage 7, the stigma has not darkened, and pollen readily germinates and grows within the style. Whether ovule degeneration is a factor in these and even further advanced flowers is work for further evaluation.
In almond, there have been conflicting reports regarding the effects of flower age and fruit set. Vezvaei and Jackson (1995) achieved the highest fruit set in newly opened flowers. In contrast, Griggs and Iwakiri (1964) indicated that older staged flowers were effective at setting fruit; flowers pollinated 3 d after emasculation had higher fruit set than those pollinated at 0 or 1 d, and fruit set remained high for 7 d. Ortega et al. (2004) obtained acceptable fruit set following pollination from day 0 to day 4 after emasculation. In these studies, morphological descriptions of floral development were not given. Environment can play an important role in the rate of flower development, emphasizing the advantage of expressing receptivity on a developmental rather than a calendar basis. In the current study, we further correlated stigma receptivity under different developmental stages with histological evaluations of almond pistils.
Papillae degeneration and the production of exudate have been associated with the beginning of stigma receptivity in tree crops such as sweet cherry and peach (Uwate and Lin, 1981; Herrero and Arbeloa, 1989). Rupture of cuticle layers during development of wet stigmas is associated with exudate production originating from epidermal and subjacent cell layers, that accumulates in the intercellular spaces of the stigmatic tissue and below the cuticle–pellicle layer of epidermal cells. The cuticle–pellicle layer is torn away with continued secretion of exudates (Konar and Linskens, 1966; Dumas et al., 1978). In sweet cherry, the primary pollen-receptive area was associated with cuticle exfoliation and papillae degeneration (Uwate and Lin, 1981). Our observations concur in that an intact cuticle layer was only observed in early stage flowers, characterized as having minimal amounts of stigmatic secretion. Degeneration of stigmatic surface cells accompanied the profuse accumulation of surface exudate in older flowers. Copious stigmatic secretions could enhance pollen hydration, germination and tube growth.
In pear, immature stigmas can support adhesion of pollen on their surface, but cannot provide a proper substrate for pollen hydration (Sanzol et al., 2003a). This could explain why almond stigmas younger than stage 6 supported very little pollen germination. Before flowers reached stage 6, insufficient exudates were produced, and little secretion occurred, suggesting that the transition of the stigma from an immature to a mature stage occurs with the acquisition of competence to support pollen hydration and germination.
More severe forms of stigma degeneration may have inhibitory consequences and result in loss of receptivity. Cessation of stigma receptivity in kiwi occurred simultaneously with papillae rupture. Pollen germination stopped when papillae degeneration and loss of cellular integrity occurred (Gonzalez et al., 1995a). However, in some cases, degeneration can be severe, yet have no apparent negative effects. The stigmatic tissue can become necrotic when the flowers are still receptive. By the later stages of flower development, the exudates can be secreted through holocrine secretion following degeneration of the protoplasts of the secretory cells (Herrero and Dickinson, 1979; Kristen et al., 1979). In the current study, no abnormal tip growth or swelling was observed for the pollen tubes growing in stage 7 flowers.
Our studies indicate that in almond, stigma receptivity is not optimal until flowers are beyond the fully open stage and exhibit flattened petals. Flowers at younger stages failed to support pollen germination, indicating that further maturation is required. Morphological evaluations confirm that stigmatic papillae elongate and exudate is produced in more mature flowers that exhibit higher percentages of pollen germination and more extensive tube growth in the style. Sufficient amounts of exudate may be prerequisite for adequate pollen function. Thus, efforts to improve fertilization for improved yield should include methods to enhance pollination in both younger and older flowers. The current results show that the stigma is still receptive, and fruit and nut set are maintained even in flowers at the stage when petals are abscising. This information is especially valuable to growers when they need to decide the time period for effective honeybee pollination.
Acknowledgments
We thank Gwen Hirsch for her technical assistance, and Paramount Farming Company for supplying financial support, plant material and field facilities. We particularly thank Joseph MacIlvaine and Nadav Ravid for their insightful discussions and contributions to this project.
LITERATURE CITED
- Burgos L, Egea J. 1993. Apricot embryo-sac development in relation to fruit-set. Journal of Horticultural Science 68: 203–208. [Google Scholar]
- Cerovic R, Ruzic D. 1992. Senescence of ovules at different temperatures and their effect on the behavior of pollen tubes in sour cherry. Scientia Horticulturae 51: 321–327. [Google Scholar]
- Connell JH. 2000. Pollination of almonds: practices and problems. HortTechnology 10: 116–119. [Google Scholar]
- Cousin M, El Maataoui M. 1998. Female reproductive organs in self-compatible almond (Prunus dulcis (Mill.) D.A. Webb) Lauranne and fertilization patterns. Scientia Horticulturae 72: 287–297. [Google Scholar]
- Dumas C, Rougier M, Zandonella P, Ciampolini F, Cresti M, Pacini E. 1978. Secretory stigma in Lycopersicum peruvianum Mill—ontogenesis and glandular activity. Protoplasma 96: 173–187. [Google Scholar]
- Egea J, Burgos L. 1992. Effective pollination period as related to stigma receptivity in apricot. Scientia Horticulturae 52: 77–83. [Google Scholar]
- Egea J, Burgos L, Garcia JE, Egea L. 1991. Stigma receptivity and style performance in several apricot cultivars. Journal of Horticultural Science 66: 19–25. [Google Scholar]
- Furukawa Y, Bukovac MJ. 1989. Embryo sac development in sour cherry during the pollination period as related to fruit-set. HortScience 24: 1005–1008. [Google Scholar]
- Gary NE, Witherell PC, Martson JM. 1976. The inter- and intra-orchard distribution of honeybees during almond pollination. Journal of Apicultural Research 15: 43–50. [Google Scholar]
- Gonzalez MV, Coque M, Herrero M. 1995a. Papillar integrity as an indicator of stigmatic receptivity in kiwifruit (Actinidia deliciosa). Journal of Experimental Botany 46: 263–269. [Google Scholar]
- Gonzalez MV, Coque M, Herrero M. 1995b. Stigmatic receptivity limits the effective pollination period in kiwifruit. Journal of the American Society for Horticultural Science 120: 199–202. [Google Scholar]
- Griggs WH, Iwakiri BT. 1964. Timing is critical for effective cross pollination of almond flowers. California Agriculture 18: 6–7. [Google Scholar]
- Guerrero-Prieto VM, Vasilakakis MD, Lombard PB. 1985. Factors controlling fruit-set of Napoleon sweet cherry in western Oregon. HortScience 20: 913–914. [Google Scholar]
- Herrero M. 1983. Factors affecting fruit set in ‘Agua de Aranjuez’ pear. Acta Horticulturae 139: 91–96. [Google Scholar]
- Herrero M, Arbeloa A. 1989. Influence of the pistil on pollen-tube kinetics in peach (Prunus persica). American Journal of Botany 76: 1441–1447. [Google Scholar]
- Herrero M, Dickinson HG. 1979. Pollen–pistil incompatibility in Petunia hybrida—changes in the pistil following compatible and incompatible intraspecific crosses. Journal of Cell Science 36: 1–18. [DOI] [PubMed] [Google Scholar]
- Heslop-Harrison Y. 1977. Pollen–stigma interaction—pollen-tube penetration in Crocus. Annals of Botany 41: 913–922. [Google Scholar]
- Kalinganire A, Harwood CE, Slee MU, Simons AJ. 2000. Floral structure, stigma receptivity and pollen viability in relation to protandry and self-incompatibility in silky oak (Grevillea robusta A. Cunn.). Annals of Botany 86: 133–148. [Google Scholar]
- Konar RN, Linskens HF. 1966. Morphology and anatomy of stigma of Petunia hybrida. Planta 71: 356–371. [DOI] [PubMed] [Google Scholar]
- Kristen U, Biedermann M, Liebezeit G, Dawson R, Bohm L. 1979. Composition of stigmatic exudate and the ultrastructure of the stigma papillae in Aptenia cordifolia. European Journal of Cell Biology 19: 281–287. [PubMed] [Google Scholar]
- Ortega E, Egea J, Dicenta F. 2004. Effective pollination period in almond cultivars. HortScience 39: 19–22. [Google Scholar]
- Pimienta E, Polito VS. 1982. Ovule abortion in Nonpareil almond (Prunus dulcis [Mill] Webb, D.A.). American Journal of Botany 69: 913–920. [Google Scholar]
- Pimienta E, Polito VS. 1983. Embryo sac development in almond [Prunus dulcis (Mill) Webb, D.A.] as affected by cross-pollination, self-pollination and non-pollination. Annals of Botany 51: 469–479. [Google Scholar]
- Pimienta E, Polito VS, Kester DE. 1983. Pollen tube growth in cross-pollinated and self-pollinated Nonpareil almond. Journal of the American Society for Horticultural Science 108: 643–647. [Google Scholar]
- Postweiler K, Stosser R, Anvari SF. 1985. The effect of different temperatures on the viability of ovules in cherries. Scientia Horticulturae 25: 235–239. [Google Scholar]
- Rallo L, Martin GC, Lavee S. 1981. Relationship between abnormal embryo sac development and fruitfulness in olive. Journal of the American Society for Horticultural Science 106: 813–817. [Google Scholar]
- Sanzol J, Herrero M. 2001. The ‘effective pollination period’ in fruit trees. Scientia Horticulturae 90: 1–17. [Google Scholar]
- Sanzol J, Rallo P, Herrero M. 2003a. Asynchronous development of stigmatic receptivity in the pear (Pyrus communis, Rosaceae) flower. American Journal of Botany 90: 78–84. [DOI] [PubMed] [Google Scholar]
- Sanzol J, Rallo P, Herrero M. 2003b. Stigmatic receptivity limits the effective pollination period in ‘Agua de Aranjuez’ pear. Journal of the American Society for Horticultural Science 128: 458–462. [Google Scholar]
- Shivanna KR. 2003. Pollen–pistil interaction and fertilization. In: Pollen biology and biotechnology. Enfield, NH: Science Publisher Inc., 117.
- Socias i Company R, Alonso JM, Gomez Aparisi J. 2002. Latest advances in almond self-compatibility. Acta Horticulturae 591: 205–212. [Google Scholar]
- Sterling C. 1964. Comparative morphology of carpel in Rosaceae. 1. Prunoideae—Prunus. American Journal of Botany 51: 36–44. [Google Scholar]
- Tangmitcharoen S, Owens JN. 1997. Floral biology, pollination, pistil receptivity, and pollen tube growth of teak (Tectona grandis Linn f.). Annals of Botany 79: 227–241. [Google Scholar]
- Tomer E, Gottreich M, Gazit S. 1976. Defective ovules in avocado cultivars. Journal of the American Society for Horticultural Science 101: 620–623. [Google Scholar]
- Uwate WJ, Lin J. 1981. Development of the stigmatic surface of Prunus avium L., sweet cherry. American Journal of Botany 68: 1165–1176. [Google Scholar]
- Vezvaei A, Jackson JF. 1995. Effect of pollen parent and stages of flower development on almond nut production. Australian Journal of Experimental Agriculture 35: 109–113. [Google Scholar]
- Williams RR, Brain P, Church RM, Flook VA. 1984. Flower receptivity, pollen transfer and fruit-set variations during a single flowering period of Cox Orange Pippin apple. Journal of Horticultural Science 59: 337–347. [Google Scholar]
- Yi WG, Law SE, Wetzstein HY. 2003a. Fungicide sprays can injure the stigmatic surface during receptivity in almond flowers. Annals of Botany 91: 335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi WG, Law SE, Wetzstein HY. 2003b. An in vitro study of fungicide effects on pollen germination and tube growth in almond. HortScience 38: 1086–1088. [Google Scholar]
- Young HJ, Gravitz L. 2002. The effects of stigma age on receptivity in Silene alba (Caryophyllaceae). American Journal of Botany 89: 1237–1241. [DOI] [PubMed] [Google Scholar]


