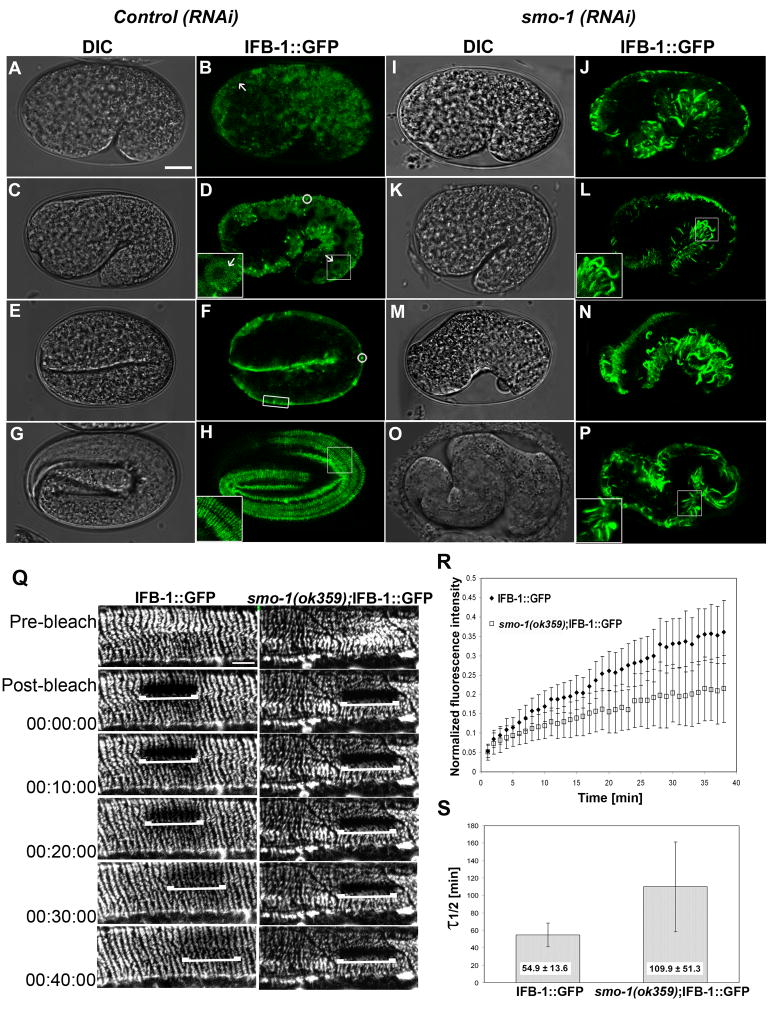
Figure 4. SUMO is required for a cytoplasmic mobile pool of IFB-1.
(A–H) DIC and expression pattern of IFB-1::GFP during elongation in wild-type embryos. (A–B) Cytoplasmic expression of IFB-1::GFP at the comma stage (arrow). (C–D) Cytoplasmic expression at 1.5-fold stage (arrow, inset). Small aggregates are circled. (E–F) The transition from cytoplasmic to localized expression in the nascent epidermal attachment sites at the 2-fold stage (box). (G–H) Fluorescence pattern of the circumferential bands of the mature epidermal attachment structures at the 3.5-fold stage (inset).
(I–P) DIC and expression pattern of IFB-1::GFP of embryos from hermaphrodites treated with smo-1(RNAi). (I–L) Formation of abnormal filaments and no cytoplasmic staining at the comma stage and 1.5-fold embryos (inset). (M–P) Embryos with severe elongation defects at estimated parallel stages exhibit abnormal pattern of filaments. Bar, 10 μm.
(Q–S) Depletion of SUMO decreases IFB-1 mobility measured by FRAP. (Q) Time-lapse micrographs of the bleached area before photobleaching (pre-bleach), immediately after photobleaching (post-bleach) and at intervals during recovery (min) of IFB-1::GFP reporter in wild-type and smo-1(ok359) animals. Bar, 5 μm. (R) Fluorescence intensities in the photobleached area are plotted against time. Values at each time point are normalized to a nonbleached area and to the fluorescence ratios before the bleach. Analysis was performed to n=12 worms from each genotype. (S) The half life time of fluorescence recovery, τ1/ 2. Mean values and standard deviations are shown. The differences between the τ1/ 2 values of the wild-type (n=12) and the smo-1(ok359) (n=12) worms are statistically significant (two sided t-test, p<0.0001). See also movies 1 and 2.