Abstract
• Background and Aims Tissue desiccation is considered to be involved in anther opening, and it is agreed that environmental humidity affects its timing. Different sources of evidence suggest that the later steps of the process (i.e. stomium opening and outward wall bending) are regulated in different ways. Anther opening was studied in Allium triquetrum under four regimes of relative humidity (RH) to analyse the effect of this parameter and to speculate about its possible regulation.
• Methods Anther histology was studied in cross-sections under a microscope. The times of visible anther opening and complete outward wall bending were recorded separately for each level of RH. Frequency distributions were plotted to express anther behaviour.
• Key Results When a longitudinal stomium breaks the anther remains closed due to adherence of walls on each side of the stomium. Anther opening occurs when the adhering walls subsequently separate. Later, the walls shrink laterally and bend outward. The anthers of the inner whorl opened during the morning of the first day of anthesis, while those of the outer whorl opened during the afternoon. Low RH (20 %) did not cause any evident acceleration of anther opening, but it did cause delay and inhibition of the opening of some anthers in the outer whorl. High RH (55 and 98 %) caused different degrees of delay and also inhibition of anther opening, but most anthers opened within the expected range of time. The time taken for outward wall bending was shortened at 20 % RH. Anther wall outward bending was inhibited at 55 % and 98 % RH.
• Conclusions Anther opening occurred at a specific moment of anther development, separated in time from stomium breakage, and seemed related to dehydration caused by reabsorption of water by contiguous tissues. Outward bending of the wall was facilitated by evaporation. Anther opening and anther wall outward bending seemed to be regulated differently in relation to water control.
Keywords: Allium triquetrum, anther opening, pollen, dehydration, filament, wall thickenings
INTRODUCTION
Anther opening implies anther wall rupture at the stomium to allow pollen release (Sanders et al., 1999). Anther opening is not a simple process because it involves several anther tissues (Bonner and Dickinson, 1989) as well as events that occur in the flower and the environment (Lisci et al., 1994; Pacini, 2000; Pacini and Hesse, 2004). Therefore, it has been studied from different perspectives. The structural features of stomium breakage have been described, the effect of different environmental parameters (e.g. temperature, relative humidity) on anther opening have been analysed, and recently the genetic regulation of anther opening has been studied (e.g. Stadler et al., 1999; Ishiguro et al., 2001).
Anther opening has always been considered a process that involves tissue desiccation (Keijzer, 1987; Bonner and Dickinson, 1989), although it is not always clear whether dehydration is provoked by reabsorption or evaporation. In Allium cepa, for instance, a reabsorption process has been suggested (Keijzer et al., 1987). It is commonly believed that anthers will not open until the weather is warm and dry enough to facilitate pollination (Faegri and van der Pijl, 1979). As a general rule, it is agreed that low relative humidities accelerate anther opening, while high relative humidities delay or inhibit the process (Linskens and Cresti, 1988; Yates and Sparks, 1993; Lisci et al., 1994). However, according to different sources of evidence, it can be deduced that the two later steps of anther opening (i.e. stomium opening and outward wall bending after Keijzer, 1987) are regulated in different ways. Stomium opening seems to be a programmed event (Stadler et al., 1999; Sanders et al., 2000; Ishiguro et al., 2001; Rieu et al., 2003; Cecchetti et al., 2004) but anther wall outward bending is, apparently, more dependant on the environment (Keijzer, 1987; Bonner and Dickinson, 1989; Bianchini and Pacini, 1996; Keijzer et al., 1996; Matsui et al., 1999, 2000). Nevertheless, although the water component seems to play a major role in anther opening, its possible internal regulation has largely been studied less (but see Bots et al., 2005).
The aim of the present study was to observe the progress of anther opening in Allium triquetrum, a longitudinal dehiscent species, under different conditions of relative humidity, in order to understand the effect of this environmental parameter and the separated regulation of the later steps of anther opening.
MATERIALS AND METHODS
The observations were made in April–May 2004 in Allium triquetrum, a longitudinal dehiscent species that grows spontaneously in the Botanical Garden of Siena. Opening flower buds were picked early in the morning (around 0800 h) and placed in water in vials closed with Parafilm (the pedicels were inserted through small holes). Two flower buds were placed in each vial. The observations were made under four different relative humidities (RH), namely 20, 40–45, 55 and 98. Three were created using saturated salt solutions in hermetically closed transparent chambers (20 % with potassium acetate, 55 % with glucose, and 98 % with potassium dichromate), as described by Winston and Bates (1960). The other RH (40–45 %) is the normal range of variation in the laboratory, which was considered a ‘control’ because anther behaviour was the same as in the natural environment (outdoors on a sunny day). The temperature was kept between 20 and 21 °C, and illumination was artificial at constant intensity. The flowers were observed for 24 h (sometimes 48 h) at the four RHs.
The anthers were observed under a stereoscopic microscope every 5–10 min through the first day of flower anthesis, from 0800–0830 h until 1700–1800 (1900) h. The time when the anther was visibly open was recorded and also the time when the anther walls were completely bent outwards. Anthers found open next morning were recorded as late-opening anthers (L). Non-opening anthers after 24 h (N/O) were also recorded. Histograms were constructed with the data. Since each anther observed was considered an independent case, the histograms show the percentages of anthers recorded in the intervals defined. A total of 189 anthers were observed in the control, 96 at 20 % RH, 69 at 55 % RH, and 69 at 98 % RH. The frequency distributions observed were regarded as trends of anther behaviour.
Individual anthers from the larger buds prior to flower opening and different moments through the first day of flower anthesis were separated. The anthers were fixed in FAA, dehydrated and embedded in Technovit 7100 resin. Sections 2 µm thick were cut on an ultramicrotome. A few anthers were embedded in Paraplast and sections 10 µm thick were produced on a rotatory microtome. All sections were stained with Toluidine Blue and observed using a light microscope. The characteristics of the cuticle were highlighted with Auramine O under UV light. Endothecial thickenings were observed in anther walls clarified with 50 % sodium hypochlorite in water (Carrizo García, 2002), using differential interference contrast. Chlorophyll/chloroplasts were detected in hand-sectioned fresh anthers at different stages under UV light.
RESULTS
Anther morphology
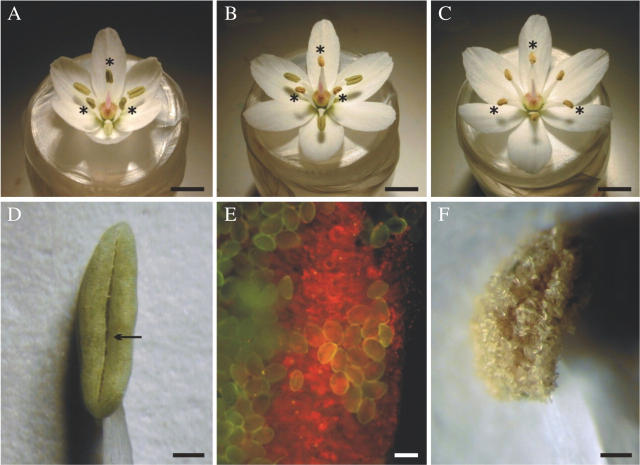
The androecium consists of six stamens arranged in two whorls (inner and outer) each with three stamens (Fig. 1A–C). The stamens of the inner whorl are slightly longer than those of the outer whorl, though the difference in the filament length decreases through the day of flower opening, from 1–1·2 mm to around 0·5 mm.
Fig. 1.
Features of the androecium and anthers of Allium triquetrum. (A–C) Flowers at different times on the first day of anthesis: (A) opening flower with all anthers closed—the stamens of the inner whorl (asterisks) are almost in vertical position and the stamens of the outer whorl are bent slightly downward; (B) flower with anthers of the inner whorl open (asterisks) and pollen completely exposed; (C) flower with all anthers open – all the stamens are at the same slightly oblique angle (stamens of inner whorl marked with asterisks); (D) lateral view of an anther open at the stomium (arrow) – note the green colour of anther wall; (E) chloroplasts (red spots) in the anther wall revealed by UV light; (F) lateral view of an anther with outwardly bent walls hidden by a disorderly mass of pollen. Scale bars: A–C = 5 mm; D and F = 0·5 mm; E = 50 µm.
The tepals extend to an almost horizontal position (Fig. 1C), a process that can take 2 h. The inner stamens are at first almost vertical (Fig. 1A), though they bend slightly downwards after the anthers have opened (Fig. 1B, C). The outer stamens first bend downwards (Fig. 1A, B) and later (near or during anther opening) slightly upwards until they reach the same position as the inner stamens (Fig. 1C).
All the anthers are equal, elongated-elliptical (Fig. 1D), dorsiventrally symmetrical (Fig. 2A). The filament is inserted in the anther base from the back. There is a sort of channel between thecae, due to the separated dorsal hemithecae, where the filament is placed. The anthers are green (Fig. 1D), even several days after they have opened, and the presence of chlorophyll was revealed by UV light throughout this period (Fig. 1E).
Fig. 2.
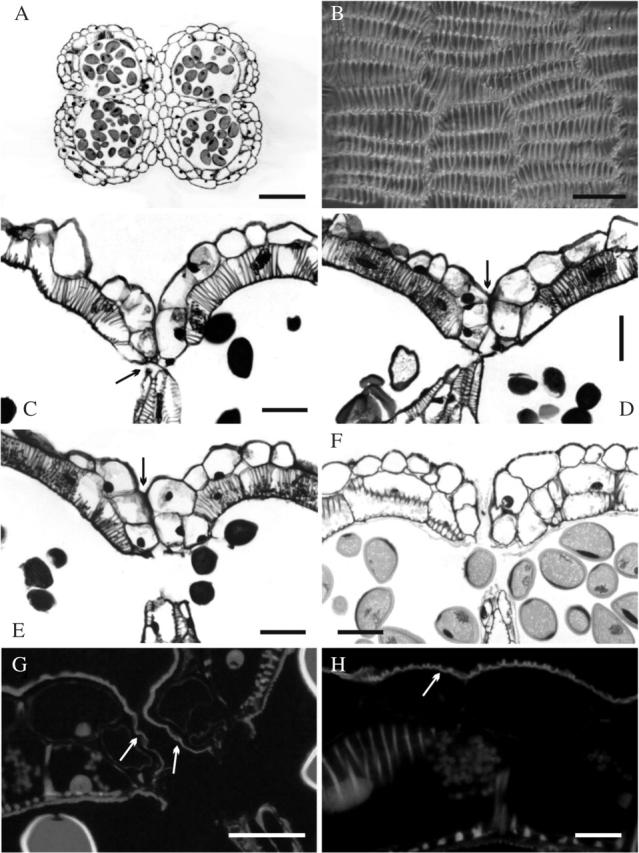
Histological features of Allium triquetrum anther. (A and C–H) Cross sections: (A) the anther has dorsiventral symmetry—note wall thickenings in the endothecium; (B) inner tangential view of the endothecium, showing tangentially elongated cells and inner bars of helical and U-shaped thickenings (some branched); (C) stomium detail before breakage in a flower bud immediately before anthesis, showing two small cells (arrow) and the septum breaking away; (D–F) successive stages during the first day of flower anthesis; (D) stomium opening—note shrinking septum and epidermal cells lining the stomium still adhering (arrow); (E) open stomium but anther still closed by epidermal cells adhering to each side of the stomium (arrow); (F) opening of the anther (i.e. open stomium, separated walls); (G) opening of the anther (i.e. open stomium, separated walls) under UV light; note smooth cuticle (arrows) lining the stomium; (H) detail of the middle part of the anther wall under UV light, showing ridged cuticle, which is common on the epidermis. Stains: A and C–F, Toluidine Blue; G and H, Auramine O. Scale bars: A = 200 µm; B–G = 50 µm; (H) 15 µm.
Anther histology
The anther wall consisted of an epidermis (without stomata) and an endothecium with cells which have wall thickenings (Fig. 2B–F). In tangential view, the cells of the endothecium were laterally elongated, with a mixed array of helical and U-shaped thickenings connected by branches (Fig. 2B). There were also thickenings in the cells of the septum (Fig. 2C–F) and the connective tissue surrounding the loculus.
Two small epidermal cells formed the stomium (Fig. 2C) that opens in advanced flower buds immediately prior to flower opening. The late septum shrinkage and rupture seemed to contribute to breaking the stomium (Fig. 2D). Even when the stomium was open, the anthers remained sealed by the epidermal cells lining the stomium (Fig. 2D, E). The anther wall bent inward making it appear that the epidermal cells on both sides of the stomium seemed to adhere to each other (Figs. 2C–E), hiding the open stomium and keeping the anther closed (Fig. 2E). These features of the stomium breakage and epidermal adherence were observed in anthers of the inner and the outer whorls of stamens at the same time. No special features were revealed in the cuticle of the epidermal cells that kept the anther closed, except that it was smooth (Fig. 2F, G), whereas it was ridged in the rest of the anther wall (Fig. 2H). Cuticle thickness was uniform across the anther (Fig. 2A). At a certain point during anthesis, the epidermal cells lining the stomium detached showing the open stomium (Fig. 2A, F, G). This step corresponds to the moment of visible anther opening (Fig. 1D) recorded in the timing measurement. The anther wall then continued bending outward.
Anther opening
Opening is through a longitudinal slit in each lateral surface of the anther (Fig. 1D). The anther walls initially bend slightly inward at the stomium (Figs 2C–E and 3A), and afterwards the walls separate from each other to open the anther (Figs 2A, F and 3B). The anthers gradually change from a bright green to a pale green colour with a dry appearance, which may indicate that some parts are dehydrating. Once the anther is opened along its whole length, the walls start to shrink and bend outward (Fig. 3C). The ventral walls bend until they touch each other (Fig. 3D), while the dorsal walls bend partially forming a concave surface (Fig. 3D). The pollen, while it dehydrates, slowly spreads over the walls and their borders in a disorderly manner (Figs 1F and 3D).
Fig. 3.
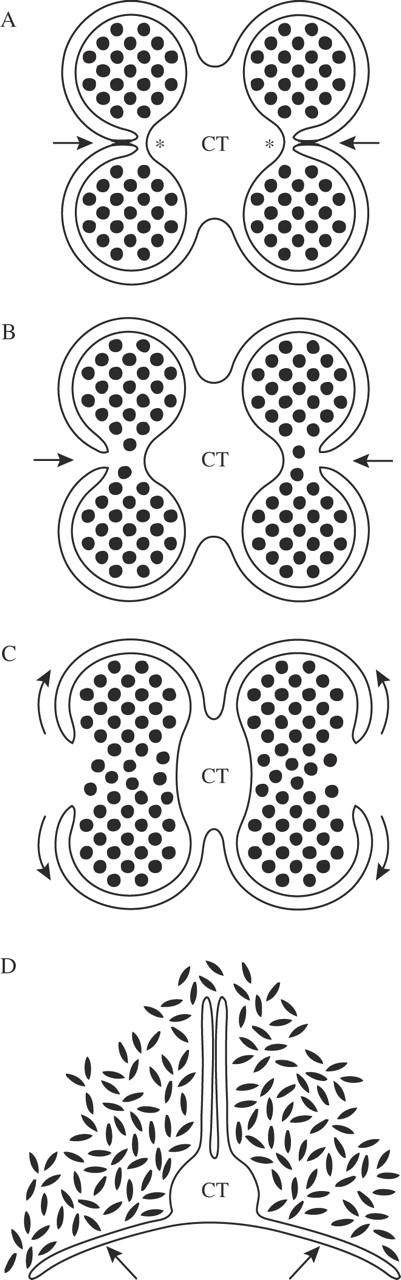
Semi-diagrammatic representation of anther opening in Allium triquetrum viewed in cross-section derived from stereomicroscopic and microscopic observations. (A) Anther walls lining the broken stomium, adhered to each other and curved inward at this site (arrows) while the septum shrinks (asterisks). Stage equivalent to that shown in Fig. 2E. (B) Anther opening; the walls split and move apart from each other showing the already broken stomium (arrows). Stage equivalent to those shown in Fig. 2A and F. (C) After the opening, the walls gradually bend outward while shrinking laterally in the directions of the arrows. (D) The ventral walls extend to lie parallel to each other, the dorsal walls bend outward to form a concave surface (arrows). The pollen, now dehydrated and coated in pollenkitt (not represented), spreads in a disorderly manner over the walls. CT, Connective tissue.
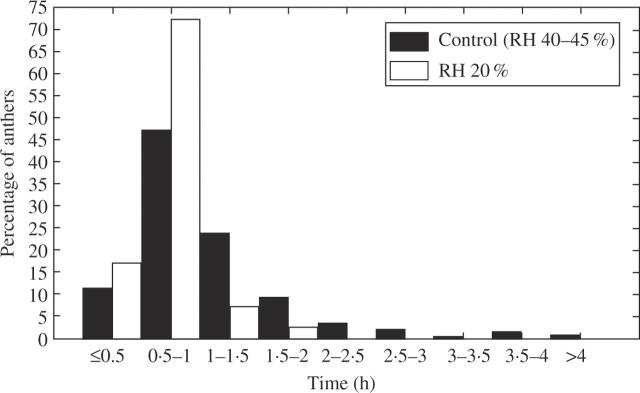
In flowers outdoors and in the control (RH 40–45 %), the anthers of the inner whorl opened first (Fig. 1B), during the morning of the first day of anthesis (Fig. 4), and they began to open when the perigonium was not yet fully extended. The anthers of the outer whorl opened later during the afternoon of the same day, sometimes after 1800–1900 h, when all the anthers of the inner whorl were already open (Fig. 4A). However, there were isolated cases of delayed or accelerated anthers that overlapped in timing with the anthers of the other whorl (Fig. 4A). Anther opening was not simultaneous within each whorl, but no specific sequence was detected. The time needed for complete outward bending of the walls varied from 30–50 min to 1·5 h in most cases, although some anthers took longer or less time (Fig. 5).
Fig. 4.
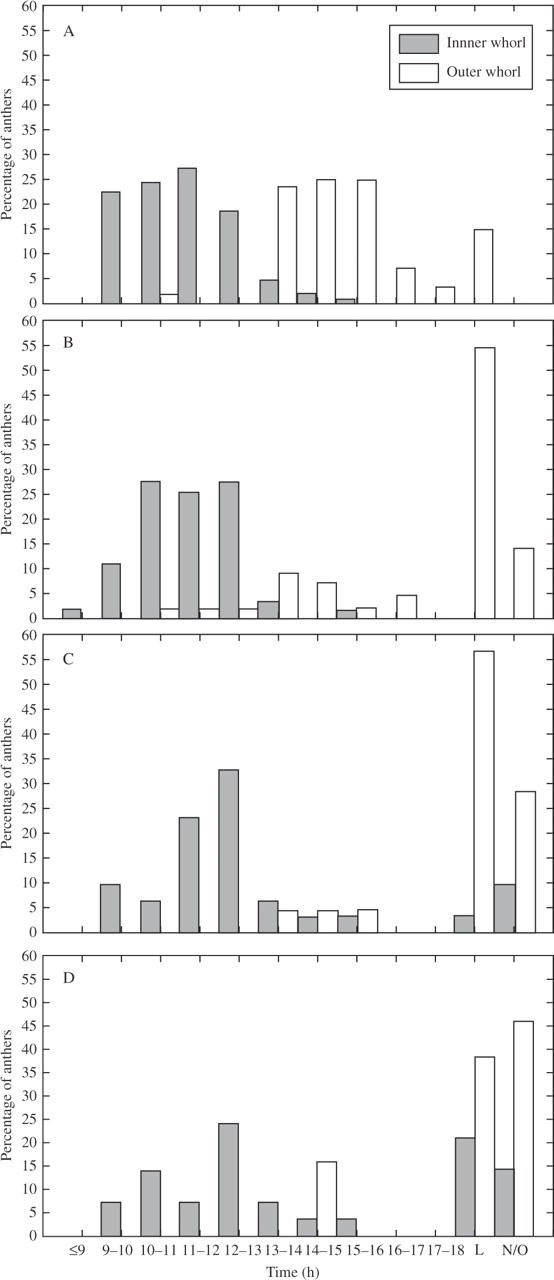
The moment of anther opening in Allium triquetrum under different environmental relative humidities (RH) during the first day of anthesis: percentages of anthers that open every hour, percentages that opened after 1800 h (L, late opening) and percentages that did not open after 24 h (N/O). (A) Control (RH 40–45 %); (B) RH 20 %; (C) RH 55 %; (D) RH 98 %.
Fig. 5.
Time taken for anther wall outward bending in Allium triquetrum. Percentages of anthers in relation to the time needed to complete outward wall bending in the control (RH 40–45 %) and at RH 20 %.
At 20 % RH, the time of anther opening did not show evident acceleration in the inner whorl, except for a few anthers that opened earlier (Fig. 4B). In the outer whorl some anthers opened slightly earlier (a small percentage of anthers that opened during the morning), but approx. 55 % opened late in the afternoon (Fig. 4B). Although the trend recognized in the control was still observable, it was slightly disturbed by early opening anthers in the outer whorl. About 14 % of anthers in the outer whorl did not open after 24 h. The time needed for outward wall bending was homogenized among anthers and shortened in many of them, most cases (about 72 %) ranging from 30 min to 1 h (Fig. 5).
At 55 % RH, different delays in anther opening were observed in the inner whorl (Fig. 4C). The highest percentage of open anthers was reached slightly later than in the control, with small percentages of anthers opening in the afternoon and about 10 % of anthers not opening at all after 24 h (Fig. 4C). The anthers of the outer whorl seemed strongly influenced by humidity for only small percentages opened in the afternoon and a high percentage (approx. 57 %) opened later. There was a higher percentage of non-opening anthers than in the inner whorl (Fig. 4C), and than at 20 % RH.
Similar effects were observed at 98 % RH. In the inner whorl, the highest percentage of open anthers appeared slightly later than in the control, but there were substantial percentages of late-opening and non-opening anthers (approx. 21 % and 14 %, respectively) (Fig. 4D). The outer whorl had a few anthers that opened in the afternoon (approx. 15 %), a higher percentage that opened later (approx. 38 %), and almost half (46 %) that had not opened after 24 h at this RH (Fig. 4D).
At RH 55 % and 98 % RH, the trend of the opening times of the two whorls of stamens was discernible, but was disturbed by late opening and non-opening anthers (Fig. 4C, D). Although most anthers opened at both these RHs, the anther walls never bent outward so that the anther aperture was inconspicuous (the anthers remained as in Fig. 1D), and the pollen continued to be enclosed inside the anther.
When anthers kept under 55 % and 98 % RH for 24 or 48 h were moved into laboratory conditions (i.e. 40–45 % RH), closed anthers opened and the walls bent outward normally, as well as in already open anthers (in approx. 70 % of cases it took between 30 min and 2 h). When anthers opened in the laboratory were kept at 98 % RH for 16 h the walls bent inward, partially closing the anther, a process that took a couple of hours.
The asynchrony of anther opening within each whorl observed in the control was maintained in the other three treatments.
DISCUSSION
Anther opening
Anther opening may occur by different mechanisms, and the sequence or pattern of opening within a flower may vary between species. For instance, anther opening in rice seems to be due to pollen swelling in the last phases of maturation (Matsui et al., 1999), while pollen has no effect on anther opening in tomato (Bonner and Dickinson, 1989). As regards the specific mechanism of stomium opening, lysis and/or mechanical forces have been proposed as the causes of stomium breakage (Keijzer, 1987; Bonner and Dickinson, 1989; Keijzer et al., 1996; Matsui et al., 1999, 2000). Although it could not be established whether there was cell lysis or cell separation in the stomium in A. triquetrum, at least from a mechanical perspective, septum shrinkage and rupture seemed to contribute to the stomium breakage, as proposed by Keijzer (1987). However, the processes of stomium breakage and anther opening are separated in time in A. triquetrum. Indeed, the anthers remained closed because the epidermis on both sides of the stomium was still intact and adhered to each other after the stomium breakage. According to the histological evidence, stomium breakage seems to occur simultaneously in both whorls of stamens, thus only the anther opening due to epidermis detachment is separated in time between them.
A certain degree of tissue dehydration seemed to facilitate anther opening (i.e. adhered walls detachment) in A. triquetrum. Dehydration does not seem to require evaporation in this species, because the anthers opened even at very high RH (98 %). If dehydration plays a role, water may be reabsorbed by contiguous tissues, perhaps the elongating flower pedicels (data not shown). Water reabsorption has also been suggested in Allium cepa (Keijzer et al., 1987) which has a similar pattern of anther opening to A. triquetrum.
Several experiences with different RHs (and other parameters) have shown that low RH accelerates anther opening while high RH may delay or inhibit the process (Keijzer, 1987; Linskens and Cresti, 1988; Yates and Sparks, 1993; Bianchini and Pacini, 1996). The present results suggest that anthers apparently do not open until a particular stage is reached in A. triquetrum. In fact, even though the stomium was already broken, anther opening was not greatly advanced at low RH (20 %) and the temporal separation between whorls was maintained. It seems that water content is controlled actively for each anther and each whorl until a particular moment. Although it seems reasonable that anthers open at a specific moment of development, which cannot be indefinitely advanced, it was not until recently that this fact was demonstrated by studies of anther opening timing in different mutants (Sanders et al., 2000; Ge et al., 2001; Ishiguro et al., 2001; Rieu et al., 2003; Cecchetti et al., 2004), although in those cases anther opening immediately follows stomium breakage. However, once that moment is reached, environmental humidity seems to affect anther opening. Indeed, if reabsorption occurs, at 55 % and 98 % RH the anthers may take water passively from the environment as well as reabsorbing internally which may delay tissue dehydration, so that anther opening takes longer to occur and may also be inhibited.
Interestingly, the anthers of the outer whorl of A. triquetrum were more affected by the environmental humidity than those of the inner whorl, perhaps because the former were exposed for longer. This fact was evidenced by increasing percentages of anthers, from RH 20 % to 98 %, that opened late or did not open. The cause of these effects is uncertain, particularly in anthers with delayed opening at RH 20 %. Some aspects of the regulation that leads to final dehydration of the anther may have been disturbed by the experimental conditions, then longer was taken to control this process, delaying or inhibiting anther opening.
The asynchrony of anther opening within each whorl and between whorls may not be related to possible microenvironments created around each anther, according to a test performed in another Allium species with a similar pattern of anther opening (data not shown). The asynchrony of anther opening was not altered when flowers were allowed to rotate, changing microenvironments constantly. This is further evidence that the timing of anther opening may be regulated internally for each anther and each whorl.
Anther wall outward bending
The width of the anther aperture has been related to the presence of thickened cells in the walls (Keijzer, 1987; Bonner and Dickinson, 1989; Bianchini and Pacini, 1996; Keijzer et al., 1996; Matsui et al., 1999, 2000). In fact, in mutants that fail to develop wall thickenings in the endothecium, the direct consequence is that anther walls do not bend outwards (Dawson et al., 1999; Steiner-Lange et al., 2003; Mitsuda et al., 2005). It has been suggested that epidermis and cells with thickenings dehydrate causing wall shrinkage and consequently outward bending (Keijzer, 1987). The dehydration of thickened cells is closely linked to environmental humidity and is apparently caused by evaporation (Keijzer, 1987; Bonner and Dickinson, 1989, 1990; Bianchini and Pacini, 1996; Matsui et al., 1999, 2000). In A. triquetrum, wall outward bending was strongly affected by RH. Low RH (20 %) shortened the time needed to complete outward bending, probably through high evaporation. On the contrary, 55 % and 98 % RH inhibited this movement, suggesting that water content could be balanced with the environment in these cases. The fact that the walls of anthers opening under 55 % and 98 % RH did bend outward in the normal way when they were moved to laboratory conditions, and that the walls of anthers opening at 40–45 % RH closed partially when placed at 98 % RH, suggests that most water movement through the wall is passive at this stage. The latter effect is similar to that one caused by rain (high RH) in Lilium philadelphicum (Edwards and Jordan, 1992). The authors explained reversible anther opening in L. philadelphicum by rehydration of the anther wall that closed the anther, which may also be the explanation for A. triquetrum. Then, water reabsorption to other tissues may decrease or cease after anther opening and free water movements through the wall may become the main factors promoting wall bending in this species.
It can be deduced from the tests presented here that anther opening and anther wall outward bending seem regulated differently regarding water movement and content.
Acknowledgments
The assistance of Laura Cresti is greatly appreciated. The suggestions of two anonymous reviewers are acknowledged. C.C.G. thanks Dr G. Barboza for permanent support and CONICET for financial help.
LITERATURE CITED
- Bianchini M, Pacini E. 1996. Explosive anther dehiscence in Ricinus communis L. involves cell wall modifications and relative humidity. International Journal of Plant Sciences 157: 739–745. [Google Scholar]
- Bonner JL, Dickinson HG. 1989. Anther dehiscence in Lycopersicon esculentum Mill. I. Structural aspects. New Phytologist 113: 97–115. [DOI] [PubMed] [Google Scholar]
- Bonner JL, Dickinson HG. 1990. Anther dehiscence in Lycopersicon esculentum. 2. Water relations. New Phytologist 115: 367–375. [DOI] [PubMed] [Google Scholar]
- Bots M, Vergeldt F, Wolters-Arts M, Weterings K, van As H, Mariani C. 2005. Aquaporins of the PIP2 class are required for efficient anther dehiscence in tobacco. Plant Physiology 137: 1049–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrizo García C. 2002. An approach to the diversity of endothecial thickenings in Solanaceae. Flora 197: 214–223. [Google Scholar]
- Cecchetti V, Pomponi M, Altamura MA, Pezzotti M, Marsilio S, D'Agneli S, et al. 2004. Expression of rolB in tobacco flowers affects the coordinated processes of anther dehiscence and style elongation. The Plant Journal 38: 512–525. [DOI] [PubMed] [Google Scholar]
- Dawson J, Sözen E, Vizir I, Van Waeyenberge S, Wilson ZA, Mulligan BJ. 1999. Characterization and genetic mapping of a mutation (ms35) which prevents anther dehiscence in Arabidopsis thaliana by affecting secondary wall thickening in the endothecium. New Phytologist 144: 213–222. [Google Scholar]
- Edwards J, Jordan JR. 1992. Reversible anther opening in Lilium philadelphicum (Liliaceae): a possible means of enhancing male fitness. American Journal of Botany 79: 144–148. [Google Scholar]
- Faegri K, van der Pijl L. 1979. The principles of pollination ecology. Oxford: Pergamon Press.
- Ge YX, Angenent GC, Dahlhaus E, Franken J, Wullems GJ, Creemers-Molenaar T. 2001. Partial silencing of the NEC1 gene results in early opening of anthers in Petunia hybrida. Molecular Genetics and Genomics 265: 414–423. [DOI] [PubMed] [Google Scholar]
- Ishiguro S, Kawai-Oda A, Ueda J, Nishida I, Okada K. 2001. The DEFECTIVE IN ANTHER DEHISCENCE1 gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence, and flower opening in Arabidopsis. The Plant Cell 13: 2191–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keijzer CJ. 1987. The processes of anther dehiscence and pollen dispersal. I. The opening mechanism of longitudinally dehiscing anthers. New Phytologist 105: 487–498. [DOI] [PubMed] [Google Scholar]
- Keijzer CJ, Hoek IHS, Willemse MTM. 1987. The processes of anther dehiscence and pollen dispersal. III. The dehydration of the filament tip and the anther in three monocotyledonous species. New Phytologist 106: 281–287. [Google Scholar]
- Keijzer CJ, Leferink-ten Klooster HB, Reinders MC. 1996. The mechanics of the grass flower: anther dehiscence and pollen shedding in maize. Annals of Botany 78: 15–21. [Google Scholar]
- Linskens HF, Cresti M. 1988. The effect of temperature, humidity, and light on the dehiscence of tobacco anthers. Proceedings of the Koninklijke Nederlandse Akademie van Wetenschappen C 91: 369–375. [Google Scholar]
- Lisci M, Tanda C, Pacini E. 1994. Pollination ecophysiology of Mercurialis annua L. (Euphorbiaceae), an anemophilous species flowering all year round. Annals of Botany 74: 125–135. [Google Scholar]
- Matsui T, Omasa K, Horie T. 1999. Mechanism of anther dehiscence in rice (Oryza sativa L.). Annals of Botany 84: 501–506. [Google Scholar]
- Matsui T, Omasa K, Horie T. 2000. Rapid swelling of pollen grains in the dehiscing anther of two-rowed barley (Hordeum distichum L. emend. LAM.). Annals of Botany 85: 345–350. [Google Scholar]
- Mitsuda N, Seki M, Shinozaki K, Ohme-Tagaki M. 2005. The NAC transcription factors NST1 and NST2 of Arabidopsis regulate secondary wall thickenings and are required for anther dehiscence. The Plant Cell 17: 2993–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacini E. 2000. From anther and pollen ripening to pollen presentation. Plant Systematics and Evolution 222: 19–43. [Google Scholar]
- Pacini E, Hesse M. 2004. Cytophysiology of pollen presentation and dispersal. Flora 199: 273–285. [Google Scholar]
- Rieu I, Wolters-Arts M, Derksen J, Mariani C, Weterings K. 2003. Ethylene regulates the timing of anther dehiscence in tobacco. Planta 217: 131–137. [DOI] [PubMed] [Google Scholar]
- Sanders PM, Bui AQ, Weterings K, McIntire KN, Hsu YC, Lee PY, et al. 1999. Anther development defects in Arabidopsis thaliana male-sterile mutants. Sexual Plant Reproduction 11: 297–322. [Google Scholar]
- Sanders PM, Lee PY, Biesgen C, Boone JD, Beals TP, Weiler EW, et al. 2000. The Arabidopsis DELAYED DEHISCENCE1 gene encodes an enzyme in the jasmonic acid synthesis pathway. The Plant Cell 12: 1041–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler R, Truernit E, Gahrtz M, Sauer N. 1999. The AtSUC1 sucrose carrier may represent the osmotic driving force for anther dehiscence and pollen tube growth in Arabidopsis. The Plant Journal 19: 269–278. [DOI] [PubMed] [Google Scholar]
- Steiner-Lange S, Unte US, Eckstein L, Yang C, Wilson ZA, Schmelzer E, et al. 2003. Disruption of Arabidopsis thaliana MYB26 results in male sterility due to non-dehiscent anthers. The Plant Journal 34: 519–528. [DOI] [PubMed] [Google Scholar]
- Winston PW, Bates DH. 1960. Saturated solutions for the control of humidity in biological research. Ecology 41: 232–237. [Google Scholar]
- Yates IE, Sparks D. 1993. Environmental regulation of anther dehiscence and pollen germination in pecan. Journal of the American Society for Horticultural Science 118: 699–706. [Google Scholar]