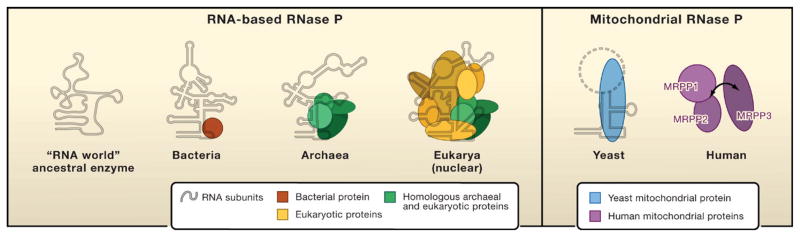
Figure 1. The Evolution of RNase P.
(Left) The compositions of characterized RNA-based RNase P enzymes from bacteria, archaea, and eukarya show an increase in protein content with increased complexity of the organism. The sites of interaction between RNase P subunits are not known in most cases and are represented schematically. The structure of the proposed ancestral RNA-only RNase P is not known and is assumed to have the critical structural elements conserved in all forms of RNase P RNA. (Right) The composition of the fully characterized mitochondrial RNase P is shown for yeast (S. cerevisiae) and human (H. sapiens). Human mtRNase P is composed only of proteins (mitochondrial RNase P proteins 1, 2, 3) (Holzmann et al., 2008). The third subunit of the human mtRNase P (MRPP3) binds to the two-protein subcomplex weakly and may associate dynamically (arrow). Although key structural elements of the RNA subunit are preserved in various yeast mtRNase P enzymes (solid line), the entire RNA structure is not well defined (dashed line).