Abstract
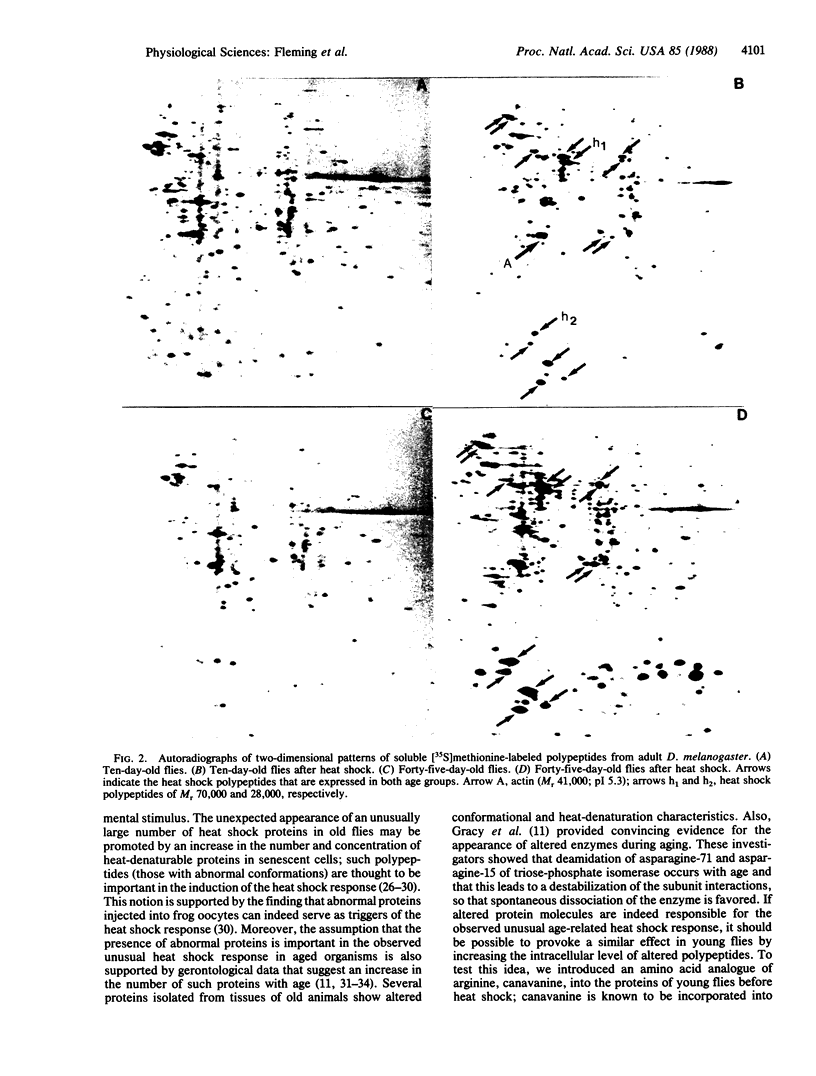
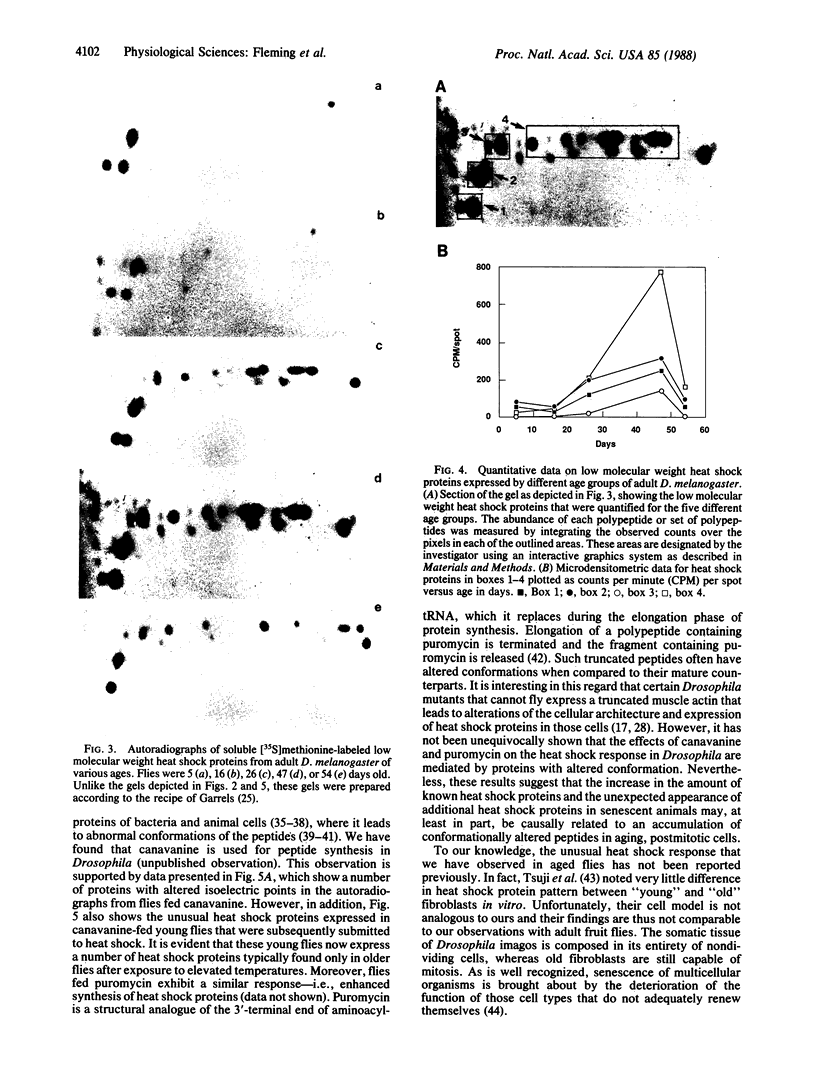
We used high-resolution two-dimensional polyacrylamide gel electrophoresis to evaluate the effect of aging on the heat shock response in Drosophila melanogaster. Although the aging process is not well understood at the molecular level, recent observations suggest that quantitative changes in gene expression occur as these fruit flies approach senescence. Such genetic alterations are in accord with our present data, which clearly show marked differences in the synthesis of heat shock proteins between young and old fruit flies. In 10-day-old flies, a heat shock of 20 min results in the expression of 14 new proteins as detectable by two-dimensional electrophoresis of [35S]methionine-labeled polypeptides, whereas identical treatment of 45-day-old flies leads to the expression of at least 50 new or highly up-regulated proteins. In addition, there is also a concomitant increase in the rate of synthesis of a number of the normal proteins in the older animals. Microdensitometric determinations of the low molecular weight heat shock polypeptides on autoradiographs of five age groups revealed that their maximum expression occurs at 47 days for a population of flies with a mean life span of 33.7 days. Moreover, a heat shock effect similar to that observed in senescent flies occurs in young flies fed canavanine, an arginine analogue, before heat shock.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLENDE C. C., ALLENDE J. E. PURIFICATION AND SUBSTRATE SPECIFICITY OF ARGINYL-RIBONUCLEIC ACID SYNTHETASE FROM RAT LIVER. J Biol Chem. 1964 Apr;239:1102–1106. [PubMed] [Google Scholar]
- Ananthan J., Goldberg A. L., Voellmy R. Abnormal proteins serve as eukaryotic stress signals and trigger the activation of heat shock genes. Science. 1986 Apr 25;232(4749):522–524. doi: 10.1126/science.3083508. [DOI] [PubMed] [Google Scholar]
- Anderson N. G., Anderson N. L. Analytical techniques for cell fractions. XXI. Two-dimensional analysis of serum and tissue proteins: multiple isoelectric focusing. Anal Biochem. 1978 Apr;85(2):331–340. doi: 10.1016/0003-2697(78)90229-4. [DOI] [PubMed] [Google Scholar]
- Ashburner M., Bonner J. J. The induction of gene activity in drosophilia by heat shock. Cell. 1979 Jun;17(2):241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Attias J., Schlesinger M. J., Schlesinger S. The effect of amino acid analogues on alkaline phosphatase formation in Escherichia coli K-12. IV. Substitution of canavanine for arginine. J Biol Chem. 1969 Jul 25;244(14):3810–3817. [PubMed] [Google Scholar]
- Bick M. D., Strehler B. L. Leucyl transfer RNA synthetase changes during soybean cotyledon senescence. Proc Natl Acad Sci U S A. 1971 Jan;68(1):224–228. doi: 10.1073/pnas.68.1.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David A. E. Assembly of the vesicular stomatitis virus envelope: incorporation of viral polypeptides into the host plasma membrane. J Mol Biol. 1973 May 5;76(1):135–148. doi: 10.1016/0022-2836(73)90085-5. [DOI] [PubMed] [Google Scholar]
- FAILLA G. The aging process and cancerogenesis. Ann N Y Acad Sci. 1958 Sep 30;71(6):1124–1140. doi: 10.1111/j.1749-6632.1958.tb46828.x. [DOI] [PubMed] [Google Scholar]
- Finley D., Ciechanover A., Varshavsky A. Thermolability of ubiquitin-activating enzyme from the mammalian cell cycle mutant ts85. Cell. 1984 May;37(1):43–55. doi: 10.1016/0092-8674(84)90299-x. [DOI] [PubMed] [Google Scholar]
- Fleming J. E., Quattrocki E., Latter G., Miquel J., Marcuson R., Zuckerkandl E., Bensch K. G. Age-dependent changes in proteins of Drosophila melanogaster. Science. 1986 Mar 7;231(4742):1157–1159. doi: 10.1126/science.3080809. [DOI] [PubMed] [Google Scholar]
- Garrels J. I. Two dimensional gel electrophoresis and computer analysis of proteins synthesized by clonal cell lines. J Biol Chem. 1979 Aug 25;254(16):7961–7977. [PubMed] [Google Scholar]
- Gershon H., Gershon D. Detection of inactive enzyme molecules in ageing organisms. Nature. 1970 Sep 19;227(5264):1214–1217. doi: 10.1038/2271214a0. [DOI] [PubMed] [Google Scholar]
- Goff S. A., Goldberg A. L. Production of abnormal proteins in E. coli stimulates transcription of lon and other heat shock genes. Cell. 1985 Jun;41(2):587–595. doi: 10.1016/s0092-8674(85)80031-3. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L. Degradation of abnormal proteins in Escherichia coli (protein breakdown-protein structure-mistranslation-amino acid analogs-puromycin). Proc Natl Acad Sci U S A. 1972 Feb;69(2):422–426. doi: 10.1073/pnas.69.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiromi Y., Hotta Y. Actin gene mutations in Drosophila; heat shock activation in the indirect flight muscles. EMBO J. 1985 Jul;4(7):1681–1687. doi: 10.1002/j.1460-2075.1985.tb03837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T. E., McCaffrey G. Programmed aging or error catastrophe? An examination by two-dimensional polyacrylamide gel electrophoresis. Mech Ageing Dev. 1985 May 31;30(3):285–297. doi: 10.1016/0047-6374(85)90118-6. [DOI] [PubMed] [Google Scholar]
- Karlik C. C., Coutu M. D., Fyrberg E. A. A nonsense mutation within the act88F actin gene disrupts myofibril formation in Drosophila indirect flight muscles. Cell. 1984 Oct;38(3):711–719. doi: 10.1016/0092-8674(84)90266-6. [DOI] [PubMed] [Google Scholar]
- Kelley P. M., Schlesinger M. J. The effect of amino acid analogues and heat shock on gene expression in chicken embryo fibroblasts. Cell. 1978 Dec;15(4):1277–1286. doi: 10.1016/0092-8674(78)90053-3. [DOI] [PubMed] [Google Scholar]
- Kirkwood T. B. Error propagation in intracellular information transfer. J Theor Biol. 1980 Feb 7;82(3):363–382. doi: 10.1016/0022-5193(80)90243-x. [DOI] [PubMed] [Google Scholar]
- Monnier V. M., Cerami A. Nonenzymatic browning in vivo: possible process for aging of long-lived proteins. Science. 1981 Jan 30;211(4481):491–493. doi: 10.1126/science.6779377. [DOI] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. Use of peptide tagging to detect proteins expressed from cloned genes: deletion mapping functional domains of Drosophila hsp 70. EMBO J. 1984 Dec 20;3(13):3087–3093. doi: 10.1002/j.1460-2075.1984.tb02263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- ORGEL L. E. The maintenance of the accuracy of protein synthesis and its relevance to ageing. Proc Natl Acad Sci U S A. 1963 Apr;49:517–521. doi: 10.1073/pnas.49.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker J., Flanagan J., Murphy J., Gallant J. On the accuracy of protein synthesis in Drosophila melanogaster. Mech Ageing Dev. 1981 Jun;16(2):127–139. doi: 10.1016/0047-6374(81)90089-0. [DOI] [PubMed] [Google Scholar]
- Pine M. J. Response of intracellular proteolysis to alteration of bacterial protein and the implications in metabolic regulation. J Bacteriol. 1967 May;93(5):1527–1533. doi: 10.1128/jb.93.5.1527-1533.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss U., Rothstein M. Age-related changes in isocitrate lyase from the free living nematode, Turbatrix aceti. J Biol Chem. 1975 Feb 10;250(3):826–830. [PubMed] [Google Scholar]
- Reiss U., Rothstein M. Heat-labile isozymes of isocitrate lyase from aging Turbatrix aceti. Biochem Biophys Res Commun. 1974 Dec 11;61(3):1012–1016. doi: 10.1016/0006-291x(74)90256-3. [DOI] [PubMed] [Google Scholar]
- Robinson A. B. Evolution and the distribution of glutaminyl and asparaginyl residues in proteins. Proc Natl Acad Sci U S A. 1974 Mar;71(3):885–888. doi: 10.1073/pnas.71.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtele C. F., Rogers P. Canavanine death in Escherichia coli. J Mol Biol. 1965 Dec;14(2):474–489. doi: 10.1016/s0022-2836(65)80197-8. [DOI] [PubMed] [Google Scholar]
- Szilard L. ON THE NATURE OF THE AGING PROCESS. Proc Natl Acad Sci U S A. 1959 Jan;45(1):30–45. doi: 10.1073/pnas.45.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji Y., Ishibashi S., Ide T. Induction of heat shock proteins in young and senescent human diploid fibroblasts. Mech Ageing Dev. 1986 Oct;36(2):155–160. doi: 10.1016/0047-6374(86)90016-3. [DOI] [PubMed] [Google Scholar]
- Wilson D. L., Hall M. E., Stone G. C. Test of some aging hypotheses using two-dimensional protein mapping. Gerontology. 1978;24(6):426–433. doi: 10.1159/000212282. [DOI] [PubMed] [Google Scholar]
- Zeelon P., Gershon H., Gershon D. Inactive enzyme molecules in aging organisms. Nematode fructose-1,6-diphosphate aldolase. Biochemistry. 1973 Apr 24;12(9):1743–1750. doi: 10.1021/bi00733a013. [DOI] [PubMed] [Google Scholar]
- von Hahn H. P. The regulation of protein synthesis in the ageing cell. Exp Gerontol. 1970 Dec;5(4):323–334. doi: 10.1016/0531-5565(70)90015-x. [DOI] [PubMed] [Google Scholar]