Table 1.
Epoxy alcohol cyclizations with various additives.
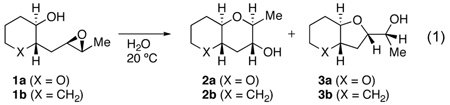 | |||||
|---|---|---|---|---|---|
| entry | X | time (h) | additive | % conversiona | 2:3a |
| 1 | O (1a) | 33 | none | 58 (4) | 11:1 (1) |
| 2 | O (1a) | 33 | CTABrb | 67 (1) | 12:1 (2) |
| 3 | O (1a) | 33 | LiCl (4M) | 88 (2) | 15:1 (1) |
| 4 | O (1a) | 33 | LiClO4 (4M) | 73 (3) | 12:1 (1) |
| 5 | O (1a) | 33 | pH 7 (1M)c | 81 (2) | 10:1 (1) |
| 6 | CH2 (1b) | 2 | none | 80 (12) | 1:1.3 (0.1) |
| 7 | CH2 (1b) | 2 | CTABrb | 71 (7) | 1:1 (0.2) |
| 8 | CH2 (1b) | 2 | LiCl (4M) | 93 (4) | 1:1.3 (0.1) |
| 9 | CH2 (1b) | 2 | LiClO4 (4M) | 81 (2) | 1:1.3 (0.1) |
| 10 | CH2 (1b) | 2 | pH 7 (1M)c | 82 (6) | 1:1.2 (0.3) |
Average of three measurements; average error in parentheses.
cetyl trimethyl ammonium bromide, 2 equiv.
K2HPO4/KH2PO4 buffer.
