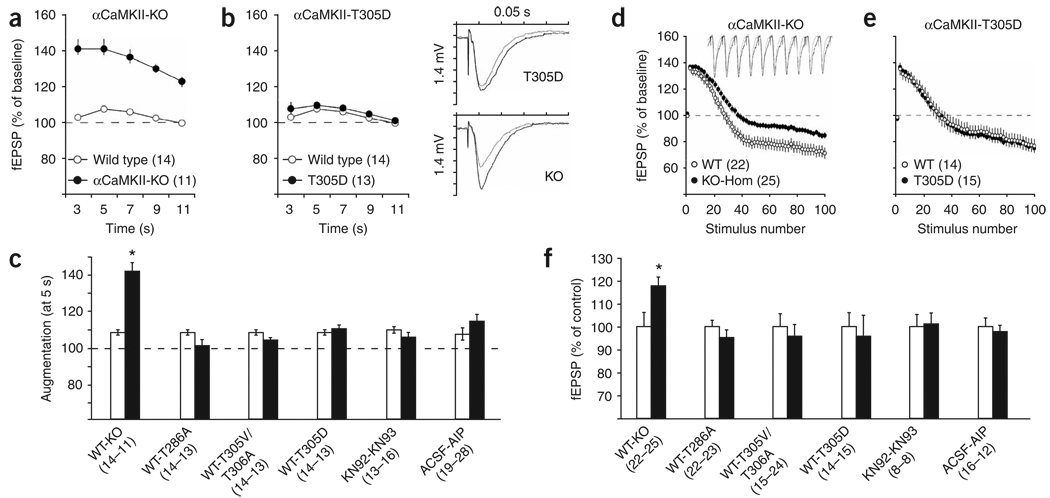
Figure 2.
Presynaptic short-term plasticity requires αCaMKII protein, but not its autophosphorylation, activation or activity. (a–c) Increased synaptic augmentation in αCaMKII-KO mutant mice was not caused by the lack of CaMKII kinase activity. fEPSP responses (normalized to pretetanus baseline) of CaMKII-KO (a) and CaMKII-T305D (b) mice were recorded at the indicated time after a 10 theta-burst tetanus. Traces are from baseline response (gray) and the response 5 s post-tetanization (black). Augmentation summary of responses obtained 5 s post-tetanus normalized to baseline is shown in c. Black bars represent mutants or drug-treated slices, white bars represent control slices as indicated. (d–f). Decreased synaptic fatigue during repetitive stimulation in αCaMKII-KO mice was not caused by the lack of αCaMKII kinase activity. (d,e) fEPSP responses (normalized against baseline) of CaMKII-KO (d) and CaMKII-T305D (e) mice were recorded during a 10-Hz tetanus. Only the first and even numbered stimuli are shown for clarity. Traces are from wild-type (gray) and αCaMKII-KO slices (black) recorded from stimulus number 21–30. Depletion summary of the last (100) stimulus of the 10-Hz train is shown in f. Black bars represent mutant or drug-treated slices, normalized against the controls as indicated (white bars, set at 100%). Numbers between brackets indicate the number of slices. Error bars indicate s.e.m.