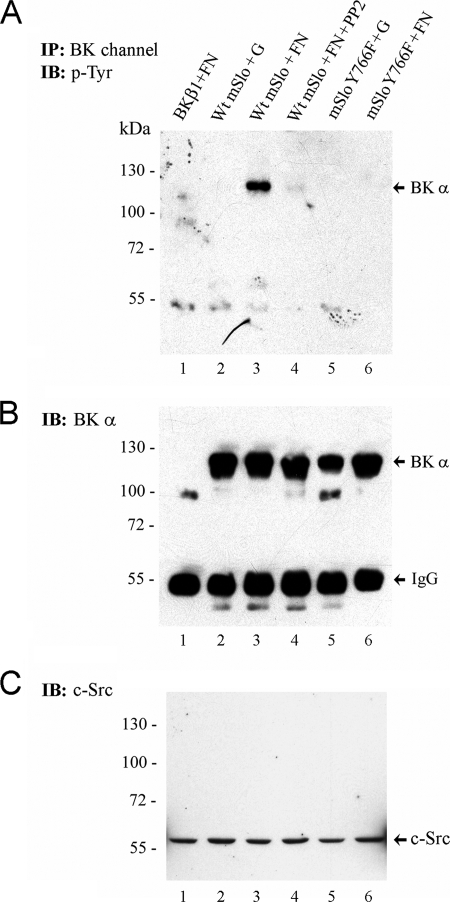
FIGURE 7.
Fibronectin induces tyrosine phosphorylation of BK channels in intact cells. Following transient expression of either WT mSlo or Y766F mSlo in HEK 293T cells, transfected cells were seeded onto tissue culture dishes coated with either porcine gelatin (G) or human FN. After 90–120 min, cells were lysed, and BK channel protein was isolated by immunoprecipitation. A, Western blot of BK channel immunoprecipitates probed with 4G10 anti-Tyr(P) Ab. B, same nitrocellulose membrane from A probed with a polyclonal antibody recognizing the BK α-subunit. Transfection of cells with cDNA encoding the BK β1-subunit served as a negative control (lane 1). In lane 4, the src kinase inhibitor PP2 (1 μm final) was added to WT mSlo-transfected cells seeded on FN-coated dishes. The positions of the monomeric BK α-subunit and IgG heavy chain are shown on the right-hand side. C, detection of endogenous c-src tyrosine kinase protein in whole lysates from transfected HEK 293T cells seeded under the same conditions as in A. An equal amount of cell lysate protein from each sample (∼40 μg) was loaded per lane. The electrophoretic positions of pre-stained molecular mass markers (size in kDa) are indicated on the left-hand side of each blot. Data are representative of two similar experiments. IB, immunoblot.