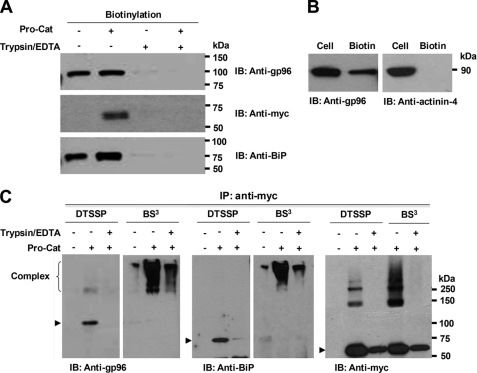
FIGURE 2.
Cell-surface localization of gp96 and BiP in HEK293F cells. Following cell-surface biotinylation (with (+) or without (−) prior trypsinization), Western blotting of streptavidin-agarose-purified proteins with the indicated antibodies demonstrates gp96, BiP, and ADAMTS9 Pro-Cat (A), but not α-actinin-4 (B) at the cell-surface. C, gp96 and BiP form complexes with Pro-Cat or furin at the cell surface. Cell-surface proteins were cross-linked with DTSSP or BS3 on ice. As a control, cell-surface proteins were eliminated by prior (DTSSP) or subsequent (BS3) treatment with trypsin. Cell lysates were immunoprecipitated with polyclonal anti-Myc, and eluted proteins were analyzed by Western blotting with anti-gp96, anti-BiP, or monoclonal anti-Myc. Note that similar high molecular weight forms (>250 kDa) with BS3 are recognized by all three antibodies, and the expected gp96, BiP, and Pro-Cat molecular species (arrowheads) disappear or are decreased when cross-linked with BS3.