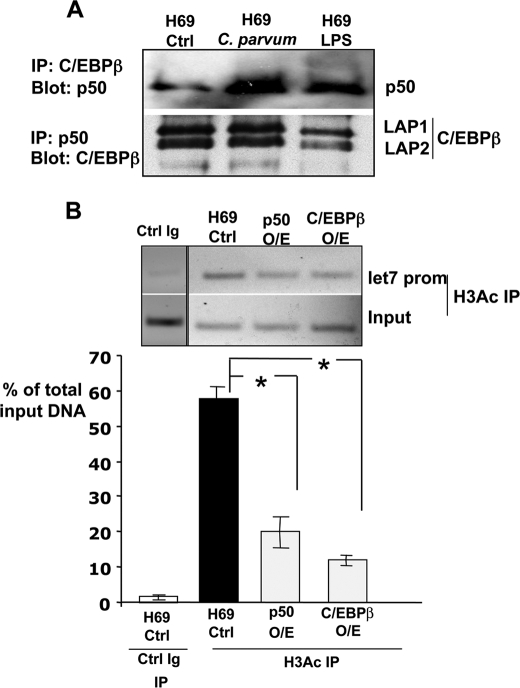
FIGURE 7.
NFκB p50 and C/EBPβ interact and overexpression of either induces let-7i promoter deacetylation. A, immunoprecipitations demonstrate the interactions between NFκB p50 and C/EBPβ. Both the immunoprecipitation of C/EBPβ with subsequent blot for p50 and the converse immunoprecipitation of p50 and blot for C/EBPβ demonstrate that these proteins physically interact in cholangiocytes. Both the Lap1 and Lap2 isoforms were detected in cholangiocytes. B, ChIP analyses for acetylated Histone H3 were performed in both control, uninfected, and in cholangiocytes overexpressing either NFκB p50 or C/EBPβ. Overexpression of either transcription factor significantly (p < 0.01) decreased the detection of acetylated Histone H3 at the let-7i promoter. The data are presented as the relative amount of the immunoprecipitated fraction compared with total input DNA. Data are represented as mean ± S.E. from three separate experiments. *, p < 0.01 compared with H69 control by ANOVA.