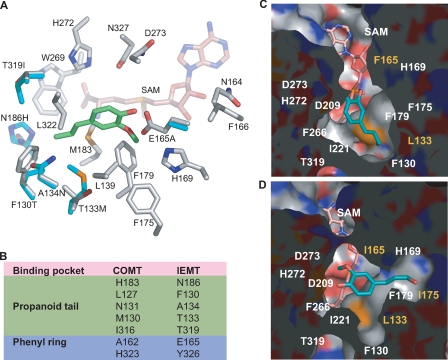
FIGURE 2.
The putative active sites of IEMT and its mutant variants. A, close-up view of the modeled IEMT active site superimposed on that of COMT; the amino acid residues of IEMT are color coded, with carbon in gray, nitrogen in blue, oxygen in red, and sulfur in yellow. Only the distinct residues of COMT in the active site are shown (carbons in cyan). The docked isoeugenol in the IEMT active site is shown in green. For clarity, one distinct residue, Tyr-326, in IEMT (His-323 in COMT) is omitted. B, the seven distinct amino acid residues in IEMT and COMT that might directly interact with the accommodated phenolics. C and D, surface representations of the active site cavity of the mutant T133L/E165F (C), and T133L/E165I/F175I (D) from the same view, illustrating the architectural changes in the binding pocket and repositioning the docked coniferyl alcohol.