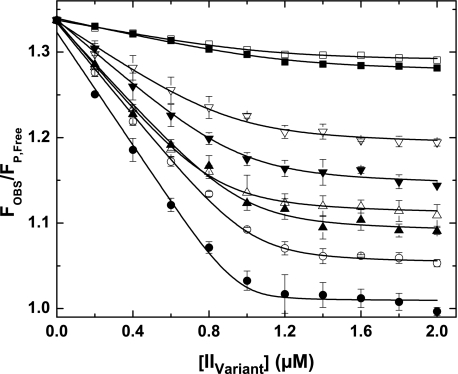
FIGURE 5.
Active site docking by prothrombin variants. Fluorescence measurements at equilibrium were determined using λEX = 320 nm, λEM = 374 nm, and reaction mixtures containing 1 μm XaS195A, 1.2 μm Va, 200 μm PCPS, 10 μm FPR-CH2Cl, 25 μm pAB, and increasing concentrations of the indicated variant. Data are presented as FOBS/FP,Free, reflecting the ratio of measured fluorescence intensity to that for pAB in solution following corrections for scatter and normalization. Titration curves are presented for IIWT (●), IIRR (○), IIRGR (▴), II-1δ (▵), II-2δ (▾), II-3δ (▿), II-4δ (■), and IIQ320 (□). With the exception of II-4δ and IIQ320 for which only one representative experiment is shown, data points and error bars denote means ± 1 S.D. from 3–5 different experiments. The lines are drawn following analysis as described under “Experimental Procedures” using the fitted constants listed in Table 2.