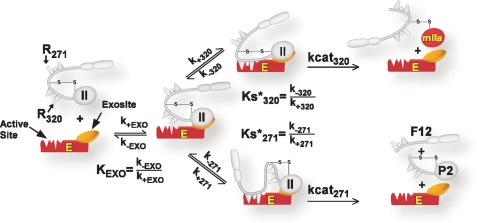
SCHEME 3.
Kinetic pathways for prothrombin binding and cleavage by prothrombinase. The annotated scheme illustrates the initial binding of prothrombin through exosite interactions with prothrombinase determined by KEXO. Exosite-bound substrate then engages the active site through one of two mutually exclusive active site docking steps. Active site engagement by Arg320, determined by Ks*320, leads to the cleavage at the 320 site and the formation of mIIa. Active site engagement by Arg271, determined by Ks*271, leads to the cleavage at the 271 site and the formation of P2 plus F12. Definition of KEXO, Ks*320, and Ks*271 in terms of rate constants is shown, and the intrinsic kcat for cleavage at either the 320 site or the 271 site is listed as kcat320 and kcat271.