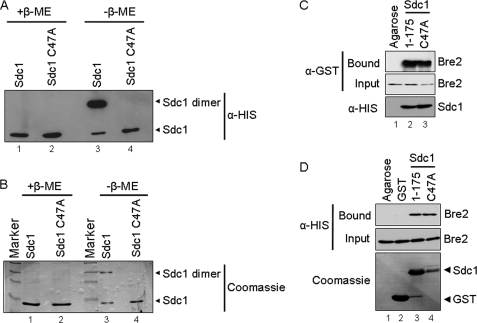
FIGURE 2.
Sdc1 forms a dimer by a disulfide interaction. A, immunoblot analysis of purified His6-Sdc1 and His6-Sdc1 C47A in the presence and absence of the reducing agent β-mercaptoethanol (β-ME). B, Coomassie Blue stain of purified His6-Sdc1 and His6-Sdc1 C47A in the presence and absence of the reducing agent β-mercaptoethanol. Bands representing Sdc1 monomer and dimer are labeled accordingly. C, nickel-agarose binding assays were performed to determine if Sdc1 dimerization affected interaction with Bre2. Purified His6-Sdc1 and His6-Sdc1 C47A were used to pull down bacterially expressed GST-Bre2 from whole cell extracts. Ni2+-NTA-agarose beads were used as a control for nonspecific binding. Input and bound fraction of GST-Bre2, His6-Sdc1, and His6-Sdc1 C47A were detected by immunoblotting with α-GST or α-His monoclonal antibodies. D, GST fusion binding assays were performed to determine if the GST-Sdc1 C47A mutation affected binding of His6-Bre2. Purified GST-Sdc1 or GST-Sdc1 C47A was used to pull down bacterially expressed His6-Bre2 from whole cell extracts. Glutathione-agarose- or GST-bound beads were used as a control for nonspecific binding. Input and bound fractions of His6-Bre2, GST, GST-Sdc1, and GST-Sdc1 C47A were detected by immunoblotting with α-His monoclonal antibodies and Coomassie Blue staining of the immunoblot.