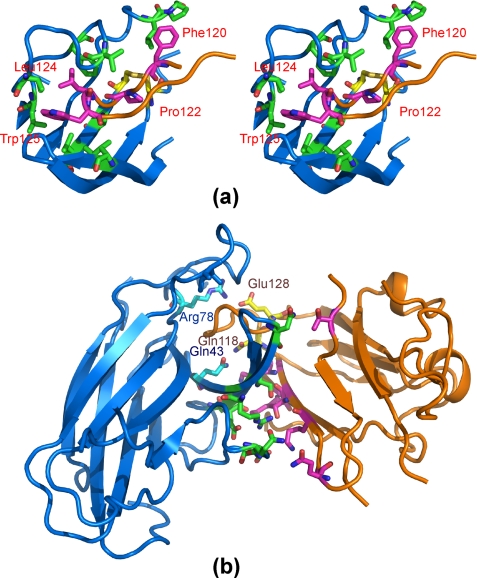
FIGURE 3.
Anatomy of the interface between the EphA4 channel and the ephrin-B2 G-H loop. a, stereo pair highlighting hydrophobic interactions between EphA4 and ephrin-B2. The ephrin-B2 hydrophobic residues in the G-H loop are labeled. b, polar interactions between EphA4 and ephrin-B2. The residues involved in polar interactions within the binding channel are labeled. A side chain hydrogen bond is formed between EphA4 Gln43 and ephrin-B2 Gln118, and a side chain salt bridge is formed between EphA4 Arg78 and ephrin-B2 Glu128. For EphA4 residues, carbon, nitrogen, and oxygen atoms are colored cyan, blue, and red, whereas for ephrin-B2 residues, they are colored pink, blue, and red, respectively. Disulfide bonds are shown in yellow.